Mclean
Thousands of people with cancer have now been tested to see if they will react negatively to certain types of chemotherapy, in what has become the biggest pharmacogenomics testing scheme in NHS history.
Around 38,000 people with cancer are initiated on fluoropyrimidine-based chemotherapy in England each year, but as many as 40% will have a genetic variant in the so-called ‘DPYD’ gene that puts them at an increased risk of a severe adverse reaction[1].
For some, these side effects can be life threatening and, in rare cases, even fatal. And that is why, in May 2020, Wales became the first UK nation to implement NHS screening for specific variants of DPYD, to enable prescribers to decide if a dose adjustment to fluoropyrimidine-based chemotherapy is needed or whether they should select an alternative treatment altogether to reduce the risk of toxicity (see Box 1).
Wales was closely followed by the NHS in Scotland and then, in August 2020, NHS England published an ‘urgent’ policy statement, mandating all patients to undergo DPYD testing prior to commencing therapy[2].
Unlike a lot of our testing, this is both urgent and high volume
Lowri Hughes, principal clinical scientist, Central and South Genomic Laboratory Hub
The introduction of DPYD testing has launched the NHS into the world of pharmacogenomics, but it has not been without its challenges — particularly during a global pandemic.
“Unlike a lot of our testing, this is both urgent and high volume,” explains Lowri Hughes, principal clinical scientist overseeing the DPYD service within the Central and South Genomic Laboratory Hub, led by Birmingham Women’s and Children NHS Foundation Trust, which has processed more than 4,000 samples since August 2020.
“It’s not very common that you get both of those things [at once], which makes it an extra challenge.”
Box 1: What is DPYD testing?
With fluoropyrimidine-based chemotherapy — which includes the drugs 5-fluorouracil (5FU), capecitabine and, less commonly, tegafur — the testing is looking for variants of the DPYD gene, which encodes an enzyme called dihydropyrimidine dehydrogenase (DPD).
This enzyme, which is mainly found in the liver, plays a central role in the metabolism of fluoropyrimidine drugs. For example, more than 80% of 5-FU is converted into its inactive form, 5-fluoro-5,6-dihydrouracil, by the DPD enzyme[3].
However, if the metabolic activity of DPD is reduced, it leads to increased concentrations of active forms of 5-FU, such as 5-fluoro-2′-deoxyuridine-5′-monophosphate. It is these active forms that increase the risk of toxicity symptoms, such as diarrhoea, hand-foot syndrome, mucositis and myelosuppression.
Certain variants in the DPYD gene can result in reduced, or even absent, DPD enzyme activity, known as DPD deficiency; a 2016 study showed that 73% of patients with the variant DPYD*2A experienced severe toxicity when treated with a full dose of fluoropyrimidine-based chemotherapy, compared with 23% of patients with the DPYD*1 variant[4].
The timing of the DPYD test depends on the type of cancer a patient has.
“If the 5-FU or capecitabine features quite heavily in the early part of [a patient’s] treatment, then they may have the test following diagnosis,” explains Sophie Harding, advanced oncology pharmacist at the Velindre Cancer Centre in Cardiff, Wales.
“Whereas if that treatment doesn’t factor into their care until later in their treatment journey, they’re likely to have the test just before the treatment [with 5-FU or capecitabine] starts.”
Testing for variants
Fluoropyrimidines are used for the treatment of a variety of cancers, but predominantly in colorectal and breast cancer. Importantly, testing for DPYD variants must happen pre-emptively, before fluoropyrimidine chemotherapy is prescribed to the patient, usually at the same time as their pre-chemotherapy bloods (see Box 2).
Nisha Shaunak, associate chief pharmacist in clinical services at Guy’s and St Thomas’ NHS Foundation Trust and pharmacy lead for South East Genomic Medicine Service Alliance (GMSA), says: “We normally [check the result] as part of the chemotherapy oncology pharmacist role when screening the prescription; we check for that variant, and make sure the dose that’s being prescribed is appropriate for that patient.”
Sometimes there are cases in which treatment is considered so urgent that it is not possible to wait for the DPYD test result. In these instances, the treatment will be started prior to testing, but at a reduced dose.
“The focus of the pathway is often on getting the initial dose right,” says Jessica Keen, a specialist oncology pharmacist at the Christie NHS Foundation Trust, and pharmacy lead for North West GMSA.
“But the pharmacists also have a role in helping with treatment selection if alternative treatments are required.”
For individuals who do experience severe toxicity, the current standard treatment is symptomatic relief and supportive care. Although, the drug uridine triacetate is approved for severe or life-threatening cases of toxicity, provided it is given within 96 hours of fluoropyrimidine being administered.
More than 160 different DPYD variants have been described in the literature but, currently, DPYD screening across the UK tests for just four of these[3].
“The ones that have been selected are based on impact on DPD; there’s good evidence to support that they reduce DPD function,” explains Keen.
“We know that there are more variants, [but] it may be that there’s not as much evidence behind them or potentially they’re at such a low frequency in the population that we don’t expect to find them.”
However, absence of the tested variants does not eliminate the risk of toxicity, because other genetic, physiological and environmental factors, such as age and comorbidities, also contribute to DPD deficiency[2].
In addition, the majority of evidence linked to the four DPYD variants currently tested for are from studies carried out in predominantly Caucasian populations. And yet, the frequency of the various DPYD variants and the associated phenotypes differs greatly between ethnic groups.
For example, in the Caucasian population, approximately 3–5% has a partial DPD deficiency compared with approximately 8% of the African-American population[3].
Currently, the estimated prevalence of DPD deficiency, whether partial or full, in the UK population is thought to be 3–8%, but some examples in the literature suggest up to 40% of fluoropyrimidine-treated patients will develop severe or life-threatening toxicity[5].
In the first year, we had 56 patients, out of 874 tests, who had their treatment adjusted to avoid side effects
Sophie Harding, advanced oncology pharmacist at the Velindre Cancer Centre, Cardiff
Sophie Harding, advanced oncology pharmacist at the Velindre Cancer Centre in Cardiff — which provides services to more than 1.5 million people — and pharmacogenomics lead at the Royal Pharmaceutical Society, says that the percentage of patients in Wales with one of the DPYD variants is around 6%.
“In the first year, we had 56 patients, out of 874 tests, who had their treatment adjusted to avoid side effects and, [in] some cases, the treatment was avoided,” says Harding.
Keen says the variants on the panel may change in the future based on emerging evidence; for example, to reflect differences between ethnic groups.
“That will be looked at in the next year,” adds Shaunak.
Box 2: The DPYD testing process
- Patient gives consent and blood sample is taken;
- Sample is sent to the laboratory to extract DNA and a genomic test is carried out;
- The results are analysed by two independent scientists. The type of analysis depends on the technology — for example, for Sanger Sequencing, scientists are looking at the DNA sequence to find the variant, whereas when using the MassARRAY system, scientists are looking for specific variants which are differentiated according to their mass (heaviest are detected last while the lightest are detected first);
- A report is sent back to the hospital and, depending on the patient’s risk of toxicity, the fluoropyrimidine therapy will either be administered as normal, the dose will be adjusted or a different therapy will be administered.
A postcode lottery
Minutes obtained by The Pharmaceutical Journal via a Freedom of Information request from a meeting of the National Genomics Board held on 8 June 2021 state that, since pharmacogenomic testing was introduced for DPYD, it has been “taken up well” in the UK.
The minutes say that oncologists initially felt testing was not needed, but that now it is available, “clinicians do use it”.
“On the whole, the uptake has been, I think, 100%,” says Keen.
However, despite the positive level of uptake, the efficiency of the service appears to be location-dependent.
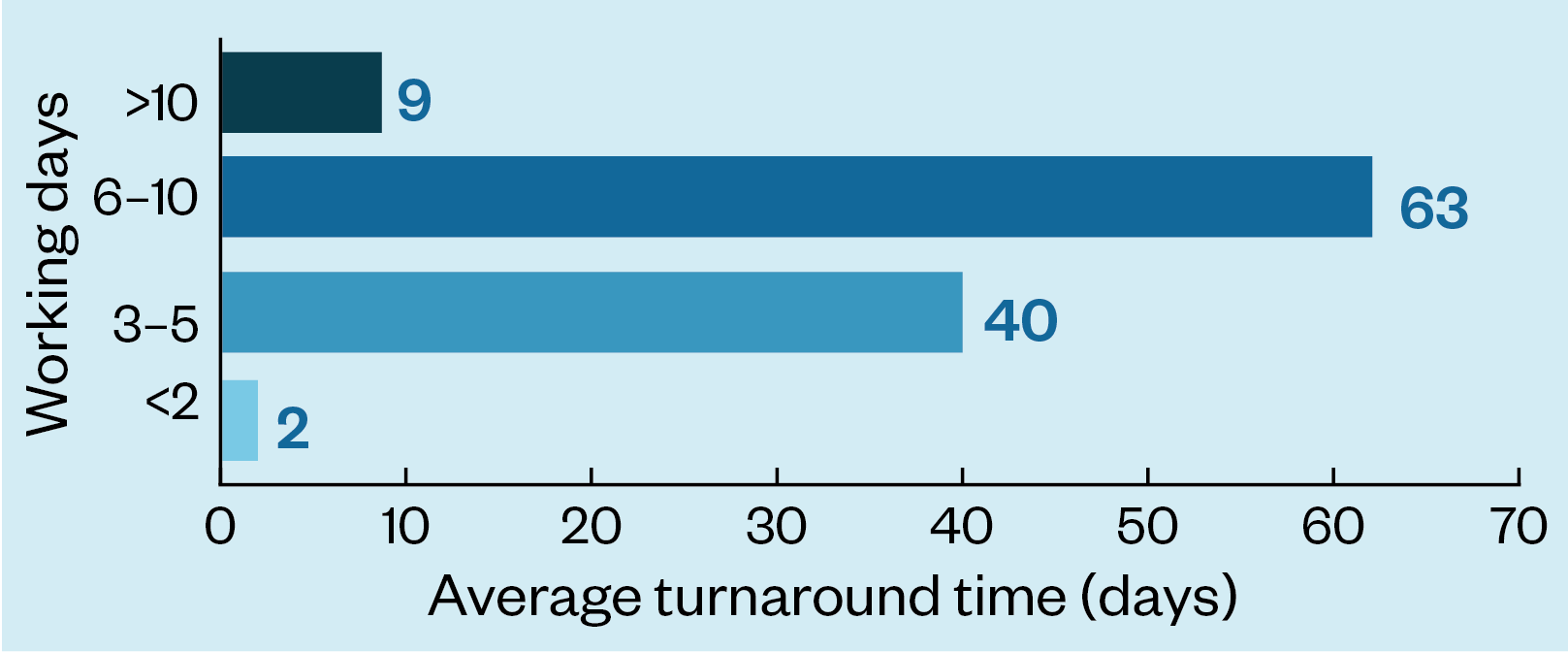
National baseline data, collected via a survey of 221 DPYD testing services and collated by the NHS GMSAs, indicate that service delivery pathways vary depending on where you are in the country. The results show that over half of those who responded to the survey (63/114) said that the average turnaround time from taking the sample to reporting the DPYD test result was six to ten working days, while over a third (40/114) said that the turnaround time was three to five working days. Nine services took longer than ten working days to report the results (see Figure 1).
“The issues are around turnaround times,” explains Shaunak. “How quickly the test was being reported after being requested — that’s the bit that needs further work.”
Hughes explains that the lack of a seven-day laboratory service means delays often occur at the weekend.
“The weekend does cause problems for us,” she explains. “But I would say most [results] go out in five days. It’s very rare things go much longer than that unless we have a technical problem or something fails.”
In Hughes’ lab, the team is now looking at a new technology to help reduce turnaround times; a direct-from-blood assay that can be delivered in a matter of hours. Although this new approach is currently undergoing validation.
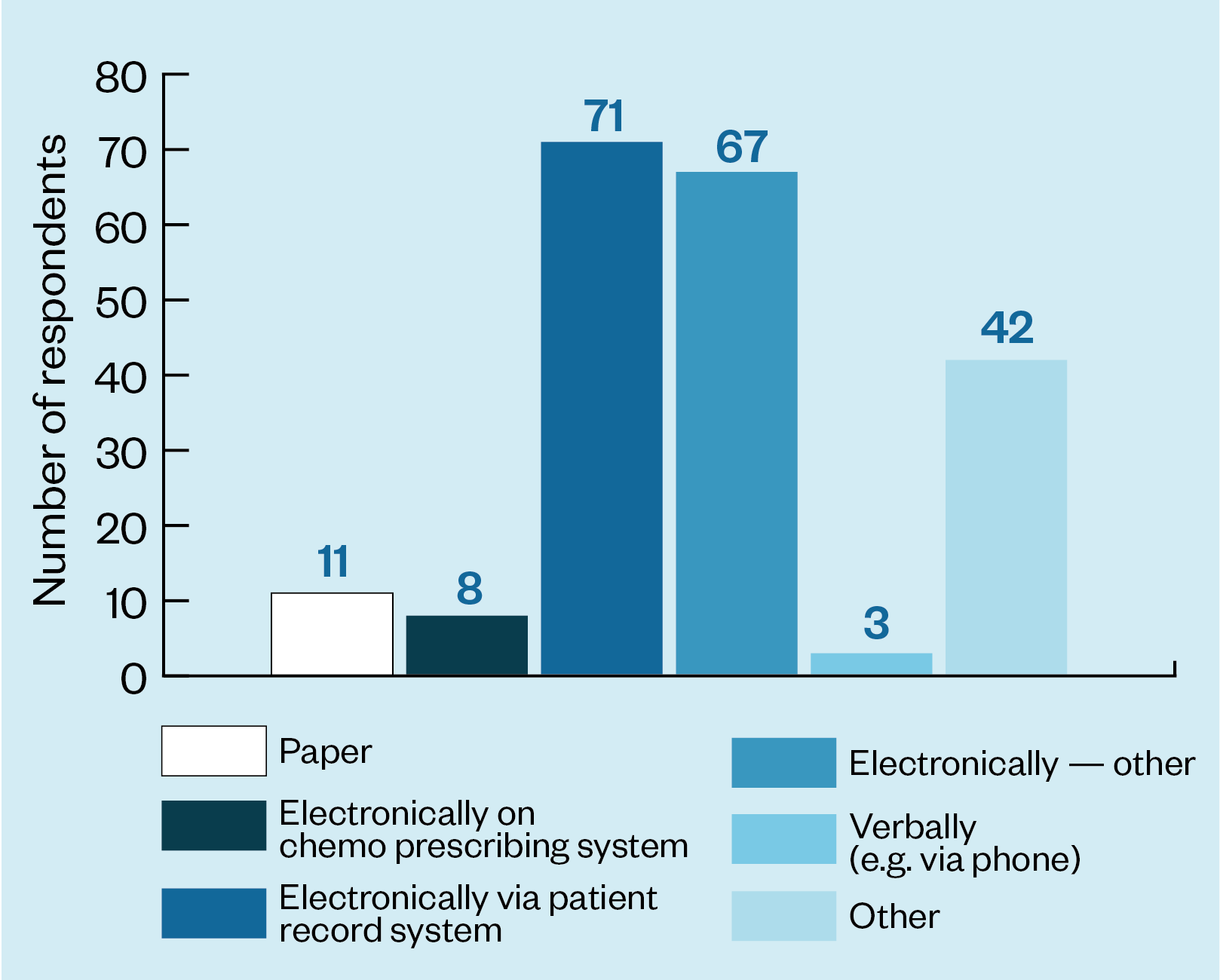
The survey showed that the method for reporting the test result also varies. The vast majority of respondents (146/202) said results was reported electronically but 11 said they reported them on paper and 3 said that results were reported verbally — for example, over the telephone (see Figure 2).
Keen explains that Shaunak and her are currently liaising with the Genomic Laboratory Hubs and the Genomics Unit at NHS England to establish a standard turnaround time for the reporting of DPYD test results.
She adds that the electronic systems available at each trust can vary, presenting challenges for the labs, which may be dealing with several different trusts at once.
At the Velindre Cancer Centre in Wales, there were some initial challenges around information governance.
“There were some issues … in regards to receiving the results and how to action those results,” says Harding.
“It’s one thing taking the test but [another thing] how that result gets to [the right] person to be able to prescribe the treatment and to reduce patient delays.
“We have an all-Wales clinical portal now which the test results are now available on, but that was an initial issue; ensuring that the pathway was complete.”
Hughes, who works with healthcare professionals from multiple hospitals, agrees that a lot of variation exists.
“Each hospital is different and each has a different setup,” she says.
“We need to make sure there’s an email account that we can send the reports to, to make sure they get to the right people. Just because the oncologist referred the patient doesn’t mean they are going to give them the result or the chemotherapy, so we need to think really carefully about where the report is most useful.
“Laying down those pathways in advance of samples coming in is very helpful.”
A gold standard
To iron out these variations, one of the essential next steps, says Shaunak, is to develop a “gold standard pathway”.
“It’s not to say that this is the way everybody should be doing it. It’s about [looking] at the gold standard exemplar pathway that we have been developing nationally and comparing that to the local processes and [asking] ‘where are the inefficiencies and what can we do to improve this?’.”
It is hoped that the pathway will be confirmed by quarter one of the next financial year.
Shaunak and Keen are currently carrying out another audit to gather more detail about the doses that patients have received during their first cycle of fluoropyrimidine chemotherapy, whether they have been tested for DPYD and if they have had a dose reduction in line with recommendations.
As part of this, they aim to link the data back to the ethnicity of patients to develop more of an understanding of the prevalence of these variants in different populations and discover more about the service from the patients’ perspective.
Reflecting on the service from her position in the lab, Hughes says that good communication is “crucial”.
“We’ve had to work with a lot of people [who] perhaps haven’t requested genomic testing before.
“And also, it’s a sort of shared learning — us educating them on our services and the complexities that we have, but also being educated by them on their patient pathways.”
At the Velindre Cancer Centre, the DPYD testing implementation project has sparked a discussions about how genomics will impact practice in the future.
“It’s opened a can of worms with personalised medicine for us,” says Harding. “We are trying to be quite proactive [and look to] the developments within genomics in the future.
“It’s an exciting thing to be part of.”
- 1NHS gives cancer patients genetic test to select best treatment. NHS England. 2020.https://www.england.nhs.uk/2020/12/nhs-cancer-patients-genetic-test/ (accessed 4 May 2022).
- 2Clinical Commissioning Urgent Policy Statement: Pharmacogenomic testing for DPYD polymorphisms with fluoropyrimidine therapies. NHS England. 2020.https://www.england.nhs.uk/publication/clinical-commissioning-urgent-policy-statement-pharmacogenomic-testing-for-dpyd-polymorphisms-with-fluoropyrimidine-therapies/ (accessed 4 May 2022).
- 3Lunenburg CATC, van der Wouden CH, Nijenhuis M, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene–drug interaction of DPYD and fluoropyrimidines. Eur J Hum Genet. 2019;28:508–17. doi:10.1038/s41431-019-0540-0
- 4Deenen MJ, Meulendijks D, Cats A, et al. Upfront Genotyping of DPYD*2A to Individualize Fluoropyrimidine Therapy: A Safety and Cost Analysis. JCO. 2016;34:227–34. doi:10.1200/jco.2015.63.1325
- 5Amstutz U, Henricks LM, Offer SM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin. Pharmacol. Ther. 2017;103:210–6. doi:10.1002/cpt.911