Science Photo Library / Alamy Stock Photo
After reading this article, you should be able to:
- Discuss the development of monoclonal antibodies and understand their place in multiple myeloma treatment;
- Describe the mechanism of action of monoclonal antibodies and antibody drug conjugates;
- Identify potential side effects of monoclonal antibodies and describe how to manage them.
Multiple myeloma (MM) is the second most common haematological malignancy, accounting for 1–1.8% of all cancers in Europe, and is the most common plasma cell disorder (a group of related diseases that result from a clonal proliferation of plasma cells)[1,2]. Typically, the presence of monoclonal protein (M-protein) either in the serum or urine is important for diagnosis of MM, as well as other plasma disorders. These proteins can be either complete molecules (paraprotein), light chains, or both. Diagnosis of MM is outside the scope of this learning article; however, further information can be found in National Institute for Health and Care Excellence (NICE) and British Society for Haematology guidelines[3,4].
As the UK population ages, the incidence of MM is likely to increase, with the disease predominantly affecting those aged over 60 years[2]. Age, male gender and family history of MM are known risk factors. Patients in the early stages of disease may have no or few symptoms, but as the disease progresses, a wide range of symptoms may present, including:
- Back pain;
- Bone pain;
- Bone fractures;
- Spinal cord compression;
- Anaemia;
- Repeated infections;
- Hypercalcaemia;
- Unusual bleeding;
- Hyperviscosity signs (e.g. blurred vision, headaches, dizziness);
- Weight loss;
- Poor appetite;
- Kidney failure[2].
Despite MM treatment advances, many patients eventually relapse and only 10–15% of patients achieve or exceed the expected survival in comparison to the matched general population[1]. Monoclonal antibodies have emerged as an additional treatment option for MM. These antibodies mimic natural proteins found in the body and are designed to recognise and attach to specific proteins on the surface of abnormal cancer cells, such as myeloma cells. There are various clinical benefits to using monoclonal antibodies for cancer therapy, including an immune-based mechanism and a different profile of toxicity compared to chemotherapy agents. Monoclonal antibodies also combine well with various existing regimens, such as chemotherapy and stem cell transplantation. As a growing component of MM treatment, pharmacists have an important role within the multidisciplinary team to educate patients about their drug treatment combinations, monitoring for potential adverse events and also managing supportive treatments. Additionally, for specialist and advanced prescribing pharmacists in haematology, learning more about the place of monoclonal antibodies in the treatment of MM further enables greater collaboration with consultants when choosing the best line of therapy for patients[5]. This article will provide a summary of monoclonal antibodies as a component of treatment for MM. It will also briefly consider future developments and the next generation of monoclonal antibody treatments currently in development.
Multiple myeloma treatment
Daratumumab is the first monoclonal antibody drug licensed in the UK for treatment of MM and has come to the forefront of treatment. The CASSIOPEIA trial, a phase III, open-label, randomised trial of patients aged 18–65 years with MM (n=1085), provided the clinical evidence for the use of daratumumab in combination with bortezomib, thalidomide, and dexamethasone[6,7]. At the primary data cut, 28.9% of patients (experimental arm) and 20.3% (control arm) had a stringent complete response after consolidation (odds ratio 1.60, 95% confidence interval [CI] 1.21 to 2.12)[6,7].
Daratumumab is a IgG1κ human monoclonal antibody that binds to the CD38 protein expressed on the surface of cells in a variety of haematological malignancies, including clonal plasma cells in MM and amyloid light-chain amyloidosis, as well as other cell types and tissues. Daratumumab has direct and indirect antitumour activity and a broad mechanism of action (see Figure; click here for full-size version)[8].
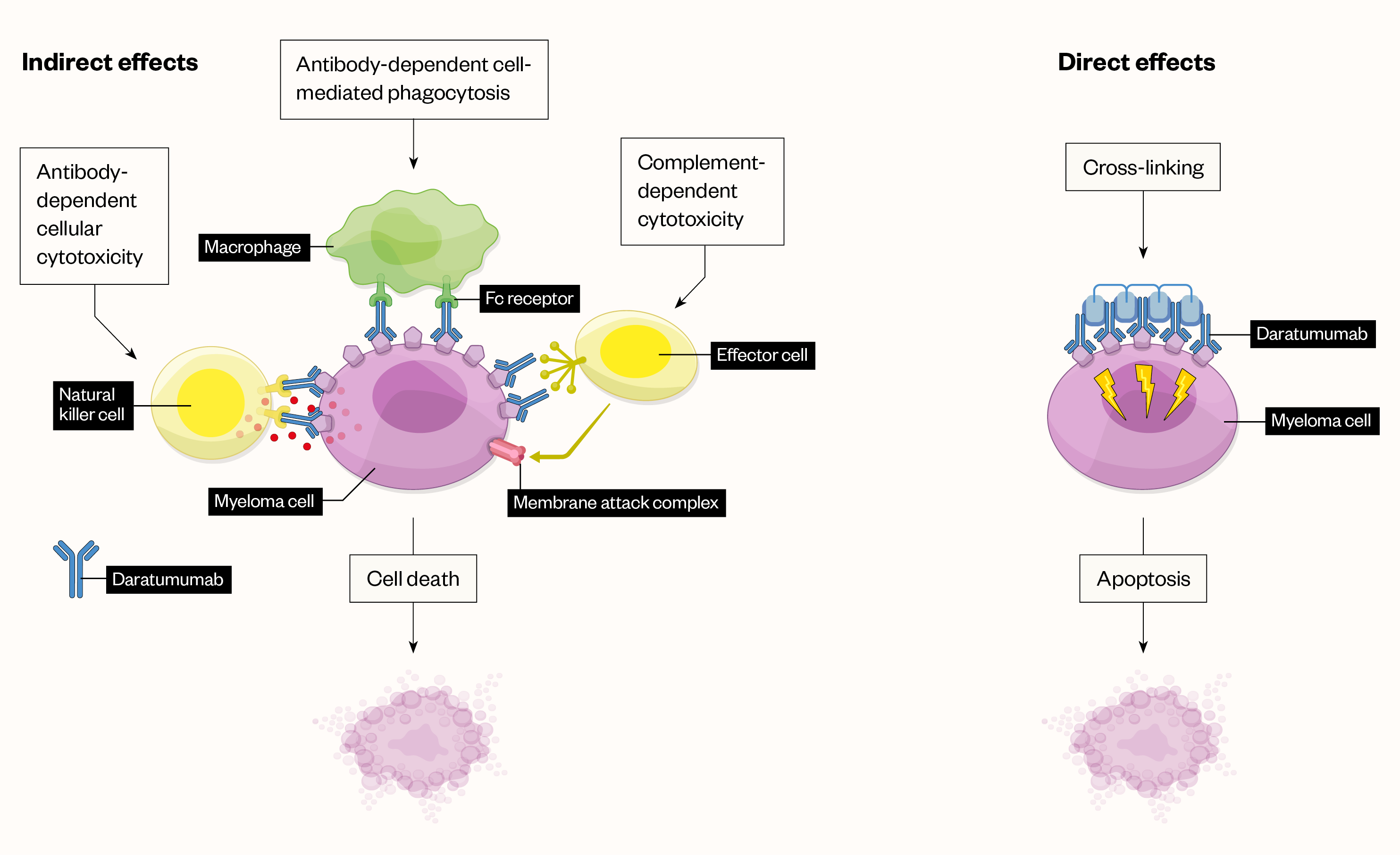
Daratumumab is now being recommended by NICE for a growing number of treatment scenarios and stages of therapy. The NICE guidance for newly diagnosed multiple myeloma currently recommends bortezomib in combination with dexamethasone, or with dexamethasone and thalidomide, for the induction treatment of adults with previously untreated multiple myeloma, who are eligible for high-dose chemotherapy with haematopoetic stem cell transplantation[3]. In 2022, the TA763 NICE technology appraisal looked at adding daratumumab to bortezomib plus thalidomide and dexamethasone before transplant (induction) and for a short time after transplant (consolidation); evidence showed good results, which led to a decision to recommend daratumumab in combination[3,6].
For patients for whom high-dose chemotherapy with stem cell transplantation is considered inappropriate, thalidomide in combination with an alkylating agent and a corticosteroid is recommended as an option for first-line treatment. Additionally, bortezomib in combination with an alkylating agent and a corticosteroid is recommended as an option for first-line treatment of multiple myeloma if high-dose chemotherapy with stem cell transplantation is not appropriate and the patient is unable to tolerate or has contraindications to thalidomide[3].
There are two different types of stem cell transplants patient can be eligible for: autologous or allogeneic stem cell transplant. Factors such as frailty and performance status are considered when assessing suitability, including comorbidities. Age and level of renal impairment alone are not suitable for assessing suitability[3]. Additional factors are also taken into consideration when assessing suitability of allogeneic stem cell transplants — for example: the degree of disease chemosensitivity; previous lines of treatment; whether a fully human leukocyte antigen-matched donor is available; how graft-versus-host disease and other complications may worsen with age; the risk of higher transplant-related mortality and morbidity versus potential long-term disease-free survival; among others[3].
Daratumumab plus bortezomib and dexamethasone is also recommended for use within the Cancer Drugs Fund as an option for treating relapsed MM in patients who have had one previous treatment[9]. The clinical evidence for daratumumab plus bortezomib and dexamethasone compared to bortezomib plus dexamethasone alone comes from the CASTOR study (open-label trial) that showed, among patients with relapsed or relapsed and refractory MM, daratumumab in combination with bortezomib and dexamethasone resulted in significantly longer progression-free survival than bortezomib and dexamethasone alone (the 12-month rate of progression-free survival was 60.7% in the daratumumab group vs. 26.9% in the control group)[10]. Daratumumab monotherapy is recommended by NICE as an option for treating relapsed and refractory MM in adults who have had a proteasome inhibitor and an immunomodulator, and where the disease progressed on the last treatment, only if: they have daratumumab after three treatments; and the manufacturer provides it in accordance with the agreement (e.g. through patient access schemes)[11].
Other monoclonal antibodies
The emergence of monoclonal antibodies and several other new agents (e.g. bispecific T-cell engagers and chimeric antigen receptor T cells) has revolutionised the treatment of MM. These new treatment options have enabled many quadruplet regimens (containing daratumumab and others) to be developed, which are showing great promise[12]. Other monoclonal antibodies, such as elotuzumab, go even further by targeting the signalling lymphocytic activation molecule F7 (SLAMF7) (a protein on immune cells). NICE guideline panels regularly review submitted evidence on these newer drugs and further advances in the use of monoclonal antibodies in MM look likely[12].
Isatuximab
Isatuximab in combination with pomalidomide and dexamethasone (Isa-Pom-Dex) is recommended for use within the Cancer Drugs Fund as an option for treating relapsed and refractory MM in adults who have had lenalidomide and a proteasome inhibitor, and whose disease had progressed on the last treatment under certain circumstances (e.g. after three previous lines of treatment)[13]. The ICARIA-MM trial (an open-label, randomised study) compared Isa-Pom-Dex against Pom-Dex[13,14]. The results showed that the addition of Isa-Pom-Dex significantly improved progression-free survival in relapsed or refractory MM. At a median follow-up of 11.6 months (interquartile range 10.1–13.9), median progression-free survival was 11.5 months (95% CI 8.9–13.9) in the Isa–Pom–Dex group versus 6.5 months (4.5–8.3) in the Pom–Dex group (hazard ratio 0.596, 95% CI 0.44–0.81; P=0.001 by stratified log-rank test)[15].
Elotuzumab
Elotuzumab is another example of a monoclonal antibody that has been considered by NICE. Elotuzumab attaches to SLAMF7 causing the cells to attack the MM cancer cells and thereby slow down the disease. Unlike daratumumab, elotuzumab does not only show single-agent activity but instead shows synergistic activity when combined with drugs such as lenalidomide and dexamethasone[12]. At the time of writing, NICE was unable to recommend its use within the NHS for previously treated patients owing to insufficient evidence, although further review may occur in the future as the evidence base develops[15].
Antibody drug conjugates and future developments
The future of antibody-drug conjugates (ADCs), comprising a monoclonal antibody linked to a cytotoxic drug, is another exciting avenue of active research[12,16]. The use of belantamab mafodotin, the first-in-class humanised IgG ADC directed against B-cell maturation antigen (BCMA) on the MM cell surface, is laying the foundations of this therapy. Belantamab mafodotin is a humanised IgG1κ monoclonal antibody conjugated with a cytotoxic agent, maleimidocaproyl monomethyl auristatin F. It binds to cell surface BCMA and is rapidly internalised. Once inside the tumour cell, the cytotoxic agent is released, disrupting the microtubule network, leading to cell cycle arrest and apoptosis. The antibody enhances recruitment and activation of immune effector cells, killing tumour cells by antibody-dependent cellular cytotoxicity and phagocytosis[17].
NICE is in the process of appraising the clinical and cost-effectiveness of belantamab mafodotin within its marketing authorisation for the treatment of relapsed or refractory MM after four or more therapies[18]. Belantamab mafodotin is currently available via a patient access scheme delivered by the manufacturer, providing the patient fits the agreement criteria.
Other drugs currently being studied include bispecific antibodies such as those targeting BCMA (teclistamab), GPRC5D/BCMA (talquetamab/teclistamab), FcRH5 (cevostamab), and CD19 (blinitumumab). If proven to be effective, bispecific antibodies could potentially be a more generalisable option for a greater proportion of patients[12,16]. There are also evidence reports to suggest bispecific antibodies could be effective for patients with high-risk disease, although more validating research is needed as the existing sample sizes in current trials are still small. There is also a need to study these and other upcoming drugs in the context of different cytogenetic anomalies; for example, del(17p), t(4;14) and t(14;16)), to further investigate their efficacy in these settings[12,16].
Commencing monoclonal antibody treatment and managing adverse reactions
At the protocol-building stage, pharmacists can ensure that important pre-medicines are listed, such as chlorphenamine, paracetamol and hydrocortisone/dexamethasone. Subsequently at the screening stage, pharmacists check to ensure these pre-medicines have been prescribed accordingly. For inpatient regimens, the transcribing of the pre- and post-medicines into the electronic/paper drug charts is vital (note that transcribing practice may vary across different NHS trusts).
Prior to commencing treatment for MM, baseline tests are required as per protocol including virology, full blood count, urea and electrolytes, and liver function tests; thereafter periodically following the treatment protocol guidance. Where appropriate, blood samples should be sent to a transfusion laboratory prior to commencement of therapy as red cell phenotyping may be required. This is because of the potential for crossmatching to fail (owing to binding of daratumumab to red cells)[5].
Prescribing specialist pharmacists running clinics, would also be reviewing disease markers including paraproteins and serum-free light chain assays to assess treatment efficacy, alongside carrying out treatment toxicity checks. Other tests that may be required for staging, informing treatment and assessing response include: calcium, albumin, uric acid, c-reactive protein (CRP), plasma viscosity, serum protein electrophoresis and immunofixation for quantification of monoclonal protein and immunoglobulins, β2 microglobulin, lactate dehydrogenase, bone marrow biopsy, myeloma fluorescence in situ hybridization, clotting screen, and others as per protocols[5].
With the use of monoclonal antibodies, there is potential for patients to experience hypersensitivity reactions, with infusion-related reactions (IRRs) being common. In clinical studies for daratumumab, approximately 9% of patients experienced an IRR; most of which occurred after the first injection and were grade 1–2[19]. Signs and symptoms of IRR may include respiratory symptoms, such as nasal congestion, cough, throat irritation, allergic rhinitis and wheezing, as well as pyrexia, chest pain, pruritus, chills, vomiting, nausea and hypotension. Severe reactions have occurred, including bronchospasm, hypoxia, dyspnoea, hypertension and tachycardia[19]. For subsequent injections, IRR occurrence was seen in 1% of patients[19]. The risk of IRRs also applies to other monoclonal antibodies.
Pharmacists can help manage and reduce the risk of these reactions by ensuring supportive medicines are prescribed. They are well placed to understand the side effects of monoclonal antibodies, immunomodulatory drugs, proteasome inhibitors and supportive care treatments, such as oral steroids (and their impact on other comorbidities, such as mental health and diabetes), anticoagulants, calcium and vitamin D supplements, as well as prophylactic antivirals, antifungals and antibiotics. Community pharmacists may be informed by the patient of any additional treatments, particularly when conducting medicines use reviews, and it is important to ensure adherence, compliance, side effects and interactions are reviewed during these consultations.
If patients are on combination treatments with high-dose dexamethasone, periodic blood glucose monitoring should also be considered owing to the risk of of hyperglycaemia. Additionally, hepatitis B virus (HBV) reactivation is another risk with monoclonal antibodies, hence the requirement for baseline virology requests as per treatment protocols[20]. Screening pharmacists play a role in ensuring these tests are reported prior to proceeding with treatment, unless otherwise discussed with the consultant. Patients who test positive for HBV serology should be managed accordingly (e.g. entecavir prophylaxis)[20]. Neutropenia and thrombocytopenia would also be monitored between treatment cycles and managed accordingly as per protocol. In some instances, dose reductions or treatment interruptions may be required.
The monoclonal antibody treatment landscape is evolving rapidly, not only for MM but also other haematological and oncological malignancies. It is important that pharmacists continue to update their knowledge and competencies to ensure the best care is provided to all patients.
- 1Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology. 2021;32:309–22. doi:10.1016/j.annonc.2020.11.014
- 2Pan-London Haemato-Oncology Clinical Guidelines. RM Partners West London Cancer Alliance. 2020.https://www.kingshealthpartners.org/assets/000/003/349/Pan_London_Plasma_Cell_Guidelines_Jan_2020_original.pdf (accessed Nov 2022).
- 3Myeloma: diagnosis and management. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/guidance/ng35 (accessed Nov 2022).
- 4Guidelines on the diagnosis, investigation and initial treatment of myeloma. British Society for Haematology. 2021.https://b-s-h.org.uk/guidelines/guidelines/guidelines-on-the-diagnosis-investigation-and-initial-treatment-of-myeloma (accessed Nov 2022).
- 5Gershman J. Multiple Myeloma Treatments, the Pharmacist’s Role in Patient Care Evolve. Pharmacy Times. 2021.https://www.pharmacytimes.com/view/multiple-myeloma-treatments-the-pharmacist-s-role-in-patient-care-evolve (accessed Nov 2022).
- 6Daratumumab in combination for untreated multiple myeloma when a stem cell transplant is suitable. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/TA763/chapter/1-Recommendations (accessed Nov 2022).
- 7Moreau P, Hulin C, Perrot A, et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. The Lancet Oncology. 2021;22:1378–90. doi:10.1016/s1470-2045(21)00428-9
- 8Varga C, Maglio M, Ghobrial IM, et al. Current use of monoclonal antibodies in the treatment of multiple myeloma. Br J Haematol. 2018;181:447–59. doi:10.1111/bjh.15121
- 9Daratumumab with bortezomib and dexamethasone for previously treated multiple myeloma. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ta573/chapter/1-Recommendations (accessed Nov 2022).
- 10Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375:754–66. doi:10.1056/nejmoa1606038
- 11Daratumumab monotherapy for treating relapsed and refractory multiple myeloma. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ta783 (accessed Nov 2022).
- 12Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. American J Hematol. 2022;97:1086–107. doi:10.1002/ajh.26590
- 13Isatuximab with pomalidomide and dexamethasone for treating relapsed and refractory multiple myeloma. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ta658/chapter/1-Recommendations (accessed Nov 2022).
- 14Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. The Lancet. 2019;394:2096–107. doi:10.1016/s0140-6736(19)32556-5
- 15Elotuzumab for previously treated multiple myeloma (terminated appraisal). National Institute for Health and Care Excellence. 2017.https://www.nice.org.uk/guidance/ta434 (accessed Nov 2022).
- 16Swan D, Routledge D, Harrison S. The evolving status of immunotherapies in multiple myeloma: the future role of bispecific antibodies. Br J Haematol. 2021;196:488–506. doi:10.1111/bjh.17805
- 17BLENREP 100mg powder for concentrate for solution for infusion. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/12545/smpc#PHARMACODYNAMIC_PROPS (accessed Nov 2022).
- 18Belantamab mafodotin for treating relapsed or refractory multiple myeloma after 4 or more therapies [ID2701]. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/indevelopment/gid-ta10568 (accessed Nov 2022).
- 19DARZALEX 1,800 mg solution for injection. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/11488/smpc#gref (accessed Nov 2022).
- 20Huang H, Li X, Zhu J, et al. Entecavir vs Lamivudine for Prevention of Hepatitis B Virus Reactivation Among Patients With Untreated Diffuse Large B-Cell Lymphoma Receiving R-CHOP Chemotherapy. JAMA. 2014;312:2521. doi:10.1001/jama.2014.15704