Abstract: Cannabidiol (CBD) is a non-psychoactive component of the cannabis plant that has garnered interest owing to its wide range of therapeutic qualities, where preclinical evidence has shown anti-tumour effects, as well as a potential role in the palliative care setting. Many of the signalling pathways that CBD targets modulate the hallmarks of cancer. Mechanistically, CBD elicits its activity through both cannabinoid receptor-dependent and independent pathways, in turn leading to ceramide production, endoplasmic reticulum stress, autophagy and apoptosis. Evidence has shown synergy when CBD is used in combination with standard chemotherapy drugs, which could be used to potentiate and leverage chemotherapy drugs, while also alleviating the harmful side effects normally observed in chemo-toxic regimens, making CBD an attractive molecule for adjunct therapy.
Key words: cannabidiol; endocannabinoid system; cannabinoids; phytocannabinoids; cancer; chemotherapy; drug delivery; side effects
Introduction
The endocannabinoid system (ECS) and its constituents, which include endogenous cannabinoids (i.e. endocannabinoids [naturally occurring lipid-based transmitters produced by the body]), synthetic cannabinoids and naturally occurring phytocannabinoids, such as cannabidiol (CBD), has gained tremendous interest in recent years for treating neurological- and cancer-related symptoms. Owing to the unwanted side effects of current cancer treatments, many patients have turned to cannabis-based products for medicinal use (CBPMs) to help ease cancer-related pain. CBPMs are compounds comprising CBD and/or tetrahydrocannabinol (THC) in various forms, including oils, tinctures, capsules, edibles, topicals, patches and sprays. They have shown merits in alleviating chemotherapy-induced pain, nausea, vomiting, decreased appetite, cachexia, sleep disturbances and anxiety, thereby improving quality of life in palliative care[1].
Several countries have legalised medical cannabis because of public pressure of use, despite a lack of clinical data. The UK legalised CBPMs in 2018 for epilepsy, such as Epidiolex (CBD containing); multiple sclerosis, such as Sativex (THC:CBD containing); and chemotherapy-induced peripheral neuropathy (CIPN), such as Nabilone (THC containing) and Dronabinol (CBD containing)[2–4]. In the United States, 26 states have legalised cannabis, with varying state-dependent possession of quantity[5]. In 2019, Canada legalised cannabis for both recreational and medicinal use, such as for treating chronic pain, multiple sclerosis, CIPN, post-traumatic stress disorder, neuropathic pain and migraines[5].
Pre-clinical evidence has shown that CBD is able to modulate the hallmarks of cancer and, in doing so, has also shown synergistic benefits as an adjunct to standard chemotherapy agents.
In this article, we aim to highlight the pre-clinical evidence and available clinical trial data that support CBD as a potential chemotherapy adjunct, while emphasising the need for more clinical studies to translate from the bench to bedside. This article will:
- Provide an overview of the endocannabinoid system;
- Explain the mechanistic pathways/targets of CBD as an anti-cancer agent;
- Outline the current routes of administration and delivery of CBD;
- Explore the evidence of efficacy of CBD in management of cancer/side effects.
The endocannabinoid system in cancer
The ECS is named after the plant Cannabis sativa, from which cannabinoids were initially identified[6]. It comprises endocannabinoids, enzymes and cannabinoid receptors, such as cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2; see Figure 1)[7]. The nature of the ECS highlights the fundamental role it plays in maintaining homeostasis throughout the body; in particular, its specific cannabinoid receptor localisation contributes to the governing of bodily functions, such as CB1 expression in the brain, which regulates learning, memory and reward, and CB2 found in the immune and peripheral system[6].
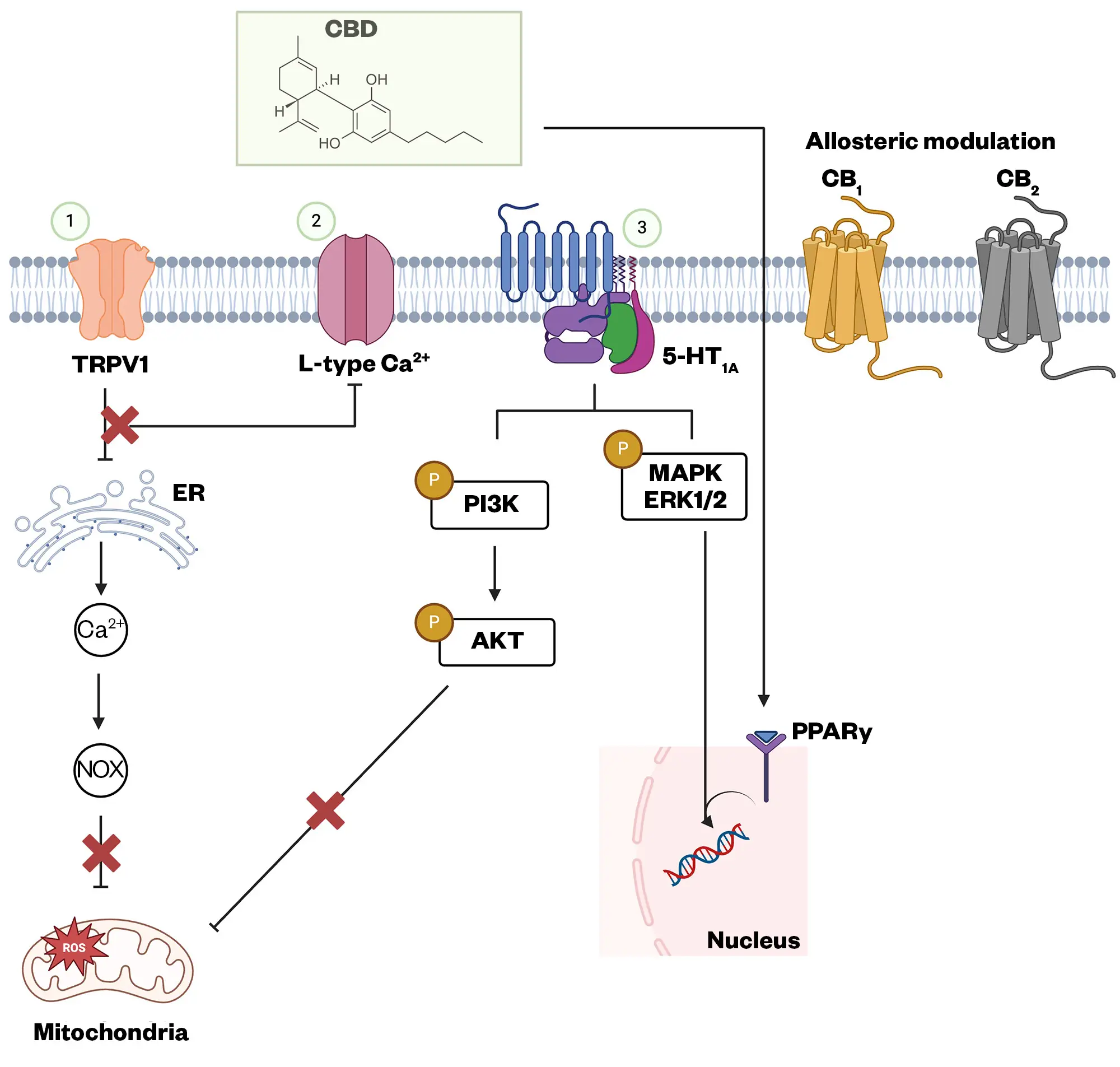
CBD can interact with several cell surface and nuclear receptors, antagonising PI3K/AKT, MAPK/ERK, and JAK/STAT pathways. CBD can also inhibit, through the PPARγ receptor, modulating DNA transcription of pro-inflammatory mediators. In addition, CBD can modify membrane and organelle calcium channels, altering intracellular signalling. CBD can indirectly exert indirect effects on CB1 and CB2 receptors
Adapted from Naya et al, 2023 (Created with BioRender.com)
In 1964, chemist Raphael Mechoulam was the first to characterise the full chemical structure of THC as the main active compound, and therefore phytocannabinoid, in cannabis[8]. Endocannabinoids occur naturally within our body and are classified as lipid mediators. These include chemical messengers, such as anandamide (AEA or arachidonoyl ethanolamide) and 2-arachidonoylglycerol(2-AG), which, in synaptic retrograde signalling, are released from postsynaptic terminals and activate CB1 receptors at presynaptic terminals (see Figure 2 for an overview of the ECS and its relevant signalling pathways)[9–18]. In some cancer models, such as breast cancer, AEA has been reported to have the anticancer effect of epithelial mesenchymal transition (EMT)[19]. The downstream receptor-mediated effects of endocannabinoids also contribute to the plasticity of the ECS, which overlaps with many of the components known to be involved in cancer progression[10].
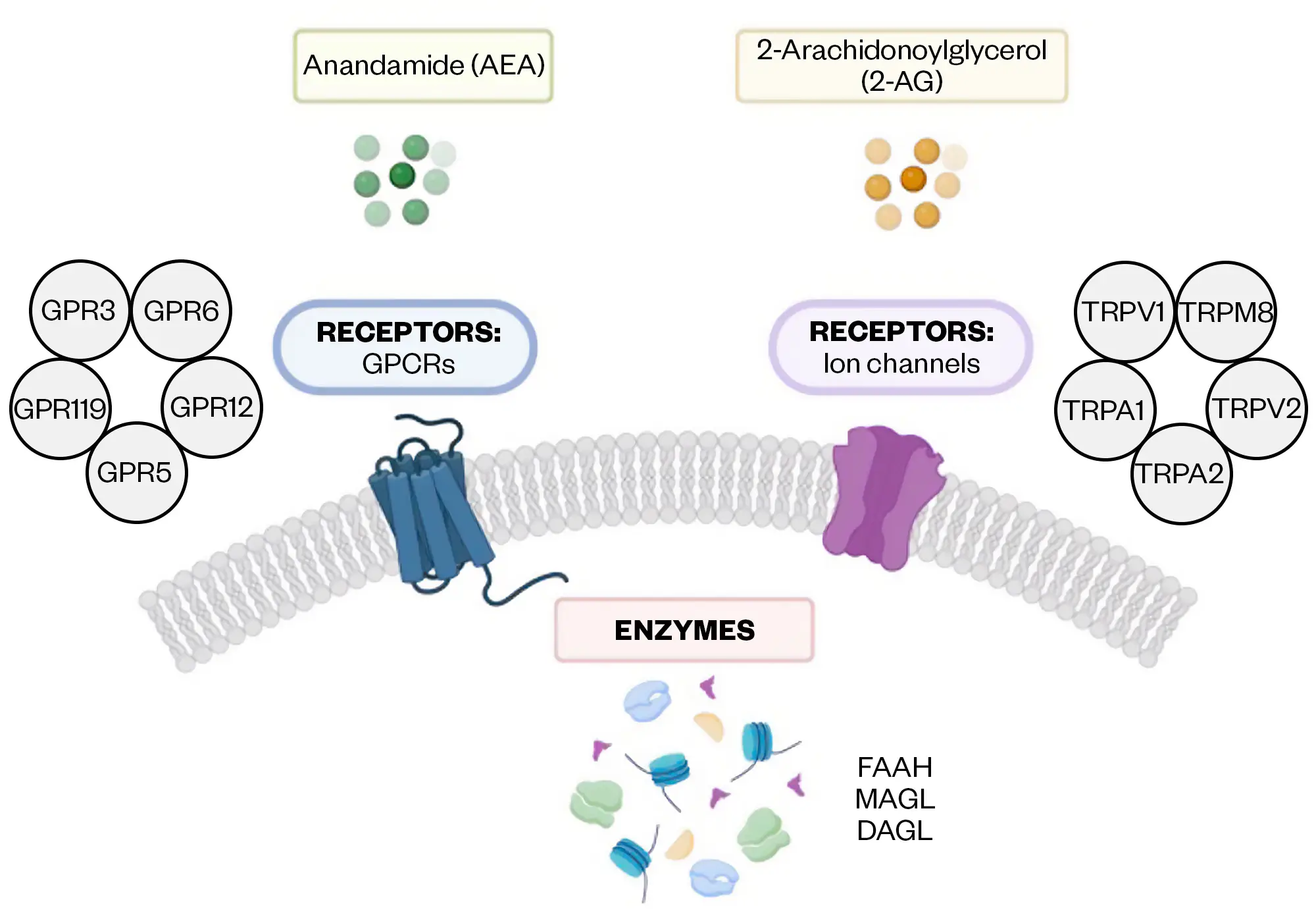
Created with BioRender.com
Mechanistic pathway/targets of CBD in cancer research in 2D and 3D models
Overall, the ECS and its components have been shown to have a role in blocking tumour growth by modulating the hallmarks of cancer; they induce apoptosis to inhibit proliferation, downregulate the vascular endothelial growth factor (VEGF) pathway, affecting angiogenesis, and dampen metastasis by inhibiting cell adhesion and migration through modifying matrix metalloproteinase (MMP2), tissue inhibitor of matrix metalloproteinase-1 (TIMP1), inhibitor of DNA binding 1 (ID1) and inducing endoplasmic reticulum (ER) stress[20–22]. There is increasing evidence that CBD can impair tumour growth through inhibiting the voltage-dependent anion channel 1 (VDAC1)[23]. CBD has been reported to mediate the anticancer effects of these pathways through CB receptors and GPR55, in addition to the de novo synthesis of ceramide[14].
Cancer signalling involves complex networks of molecular pathways that regulate fundamental cellular processes, such as growth, proliferation, differentiation and survival. Under normal cellular conditions, these signalling pathways maintain a balance, ensuring controlled behaviour[24,25]. However, in cancer, these pathways malfunction, leading to uncontrolled cell growth and tumour formation (see Figure 3)[23–26].
Cell cycle arrest
The cell cycle is a tightly regulated process that ensures the detection and repair of genetic damage for cell survival and correct division[27]. CBD can arrest the cell cycle and induce downstream apoptosis; a study investigating gastric cancer cells found that CBD could halt the cancer cells at the G0-G1 phase while upregulating the expression levels of ATM and p53 and downregulating p21, CDK2, and cyclin E protein levels[28]. While CBD and THC are the most studied phytocannabinoids, cannabinol (CBN) has recently been reported to reduce the proliferative state of liver and breast cancer cell lines HepG2 and HCC1806, respectively[29]. The authors of this study report downregulation of p21 and p27, as well as cell cycle arrest at the G1 or S phase through a decrease in expression of CDK1, CDK2 and cyclin E1[29]. This finding indicates that other phytocannabinoids may also conserve these anti-cancer effects and that more research is needed on their potential as additional alternatives/adjunct for cancer therapy.
Apoptosis
The induction of ceramide accumulation via CB receptors has been shown to lead to apoptosis in pancreatic, glioma, colon, and many other cancer cells[1,30]. Many of the cannabinoid signalling pathways result in reactive oxygen species (ROS) involvement and this has been widely observed in glioma and leukaemia[31–33]. In addition, ROS involvement is further supported by the involvement of N-acetylcysteine, a thiol antioxidant that scavenges ROS, or the NAD(P)H oxidase inhibitors that can attenuate the effects of cannabinoids[34]. CBD can interact with CB receptors that are differently expressed in neural and peripheral tissues, with transient receptor potential channels of the vanilloid type-1 (TRPV1), or directly with membrane microdomains rich in cholesterol named lipid rafts[35]. Often, the interaction of CBD with different receptor types leads to the same cell fate, even if different intracellular signalling cascades have been activated. Downstream events following ROS or ceramide induction has brought light to the involvement of ER stress. It has been observed in vivo that a high level of ROS induces ER stress, which is evidenced by the increase in levels of the specific ER-stress mediators; p8, CHOP, TRB-3 and GPR78, which activate the mitochondrial intrinsic apoptotic pathway[33]. Ceramide level increases have also been associated with ER stress in CBD-induced apoptosis in tumour cells[36,37]. The mitogen-activated protein kinase (MAPK) pathway has also been reported in numerous studies to be involved in CBD exposure[38]. Serine/threonine protein kinases are mainly involved in the pathway and act to convert extracellular stress into cellular responses, including cell cycle arrest, apoptotic cell death and cytokine production via phosphorylation[39]. The involvement of this pathway in cancer has been largely reported in the literature and the duration of the stimulus has been reported to be important for the type of cellular response, whereby a brief activation of the ERK cascade leads to cell survival and proliferation, and a long-term activation leads to apoptosis[40,41].
The PI3K/AKT pathway has been reported to be involved in intracellular pro-survival signalling and regulates cell survival, growth, proliferation, angiogenesis, cell migration and invasion[42]. The downregulation of AKT is involved in CBD’s anticancer effect and, in gastric cancer, induces cell cycle arrest, which is a consequence of AKT inhibition related to MAPK pathway activation[29]. In astrocytoma, CBD induces apoptosis in low CB receptor-expressing cells in comparison to high CB receptor levels, owing to the high levels of phosphorylated AKT present. This suggests that a high level of CB receptors, in addition to AKT, eliminates the ability of cannabinoids to induce apoptosis in astrocytoma cells[43]. The AKT pathway, therefore, stands as a potential target for clinical trials[44].
In a study investigating CBD in pancreatic cancer, the authors report anticancer effects via antagonisation of the GPR55 receptor through CBD[14]. They found that p53, a tumour suppressor gene, regulates GPR55 and modulates cell cycle and MAPK pathways[14]. Pancreatic cancer cell lines AsPc1, HPFA-II, BxPc3 and Panc1 were treated with CBD as well as an antagonist of GPR55, CID16020046 (CID), and found a reduction in cell growth with combinatorial CBD/CID and gemcitabine[14]. The study demonstrates a novel pathway by which gemcitabine may potentiate anticancer effects through inhibition of GPR55[14].
Autophagy
Autophagy is a mechanism by which unnecessary and dysfunctional components of a cell are removed and recycled via PI3K/AKT/mTOR and AMP kinase signalling; as a last resort of stress to the cell, autophagy can transition to cell death[45]. As above, ER stress can drive mitochondria-dependent apoptosis through activation of CHOP or determine cell survival by an increase in GPR78[46]. However, if the level of CHOP targeting TRB-3 significantly increases, ER stress then triggers autophagy[46,47]. In breast cancer, CBD induces ER stress and subsequent inhibition of AKT and mTOR signalling, suggesting the induction of autophagy[48]. Apoptosis and autophagy can co-exist — CBD, for example, can induce ROS production causing an inhibition of both processes; this is suggested by evidence from the effects of higher CBD concentration favouring cell death with a decreased association between beclin1 and Bcl-2, marking both autophagy- and CBD-mediated apoptosis[48].
The apoptosis and autophagy processes involved in cell death are complex, with overlapping features[49]. A third type of programmed cell death has been reported in a study investigating cannabinoids in mantle cell lymphoma[50]. The authors report a decrease in cell viability by CBD, without involvement of caspase-3, but instead cycloheximide-sensitive cytoplasmic vacuoles[50]. The lack of enhanced autophagosome formation and lysosomal contribution excludes the involvement of canonical autophagy. This indicates that there may be additional types of cell deaths activated by CBD.
Angiogenesis, invasion and metastasis
Cannabinoid receptor agonists are able to block the induction of angiogenesis by inhibition of the VEGF pathway[51]. Within this signalling cascade, cannabinoids act by targeting various elements, including VEGF receptors VEGFR1 and VEGFR2, which are downregulated upon treatment of gliomas, skin carcinomas and thyroid carcinomas[52]. Cannabinoid receptor activation in vascular endothelial cells leads to the inhibition of proliferation and migration and promotes apoptosis[51]. CB1 and CB2 agonists can reduce spontaneous metastasis of distant tumour masses in vivo and cause inhibition of adhesion, migration and invasiveness in vitro in glioma, lung and cervical cancers[53–55]. These anti-cancer effects have been reported to involve modulation of extracellular proteases, such as MMP2 and TIMP1[53,55].
Pharmacological inhibition of ceramide biosynthesis results in an anti-tumor and anti-angiogenic effect of CB1 and CB2 receptor agonists and decreases VEGF production both in vitro and in vivo in glioma[51]. Blocking ceramide biosynthesis and knock down of the p8 protein prevents inhibition of MMP2 expression and cell invasion in glioma[53]. However, CBD can reduce invasiveness and metastasis by acting independently of the CB1 and CB2 receptor, partly owing to downregulation of the helix-loop-helix transcription factor inhibitor of ID-1[56,57]. Emerging studies have proposed further pathways for the mechanism of action of cannabinoids. A recent study reports CBD could inhibit the release of exosome and microvesicles (EMV) from prostate, hepatocellular and breast cancers in vitro[58]. EMV release is associated with many diseases, such as cancer; Kosgodage et al. reported that an increase in EMV release may be the cause of chemoresistance[58]. In their study, they propose the regulatory effects of CBD on EMV biogenesis as a novel anti-cancer target, which may be used to sensitise cancer cells to chemotherapy[58].
A further 2019 study by Kosgodage et al. reported CBD-EV-mediated modulation of EV in glioblastoma[59]. Prohibitin (PHB) — an intracellular iron-binding protein — is involved in many roles, including cell metabolism, apoptosis, senescence, survival and immunity, and is crucial for mitochondrial function and integrity[60]. Increased PHB levels are associated with chemoresistance in cancers and are therefore a target of therapeutic interest. In this study, CBD was shown to reduce PHB protein levels and exchanged EV-mediated export of microRNAs to an anti-oncogenic signature in glioblastoma multiforme (GBM) cells[59].
A study investigating the effect of CBD on hypoxia inducible factor 1 subunit alpha (HIF-1α), a protein expressed in response to hypoxia that activates genes to increase oxygen, has shown that HIF-1α suppresses angiogenesis and stem cell-like properties of breast cancer by decreasing the expression of HIF-1α through the Src/von Hippel–Lindau tumour suppressor protein (VHL) interaction[61]. Meaning CBD acts to inhibit Src (Proto-oncogene tyrosine-protein kinase) activity, which decreases HIF-1α through the degradation of VHL recruitment and the direct reduction of HIF-1α protein synthesis[61].
3D models
The use of 3D cancer models to study and evaluate the potency of drugs has proven to be an efficient tool for recapitulating the tumour microenvironment (TME) to better understand the cancer-drug interaction in real time. The majority of the pre-clinical investigations into CBD’s oncological and synergistic effects with chemotherapy drugs have utilised 2D cell lines and animal models. In triple negative breast cancer (TNBC), Surapaneni et al. reported inhibitory concentrations (IC50) of <3.5mM in 2D cell lines treated with CBD; however, a greater IC50 value of 75.36mM was observed in 3D organoids derived from a mouse model[62]. Another report showed that, following 48 hours treatment, 80mM of a CBD-rich extract exhibited cytotoxic effects in pancreatic ductal adenocarcinoma spheroids greater than gemcitabine at the same concentration[63]. These findings indicate further research is needed for evaluating CBD and chemotherapy drug combinations as potential TME-modulating therapies.
Drug administration and delivery
The current routes of administration for CBD include oral formulations, transdermal, pulmonary and transmucosal[64,65]. Although oral delivery is the preferred route of administration because of its ease of manufacturing and patient preference, it comes with many challenges, including poor bioavailability, variable pharmacokinetics, dosing and side effects, possible polymorphisms and drug–drug interactions[65].
Oral formulations have shown low bioavailability, which may be owing to slow and erratic absorption and degradation in the stomach[66]. The maximum plasma concentrations are usually obtained following one to two hours and can last up to six hours[65]. Self-emulsifying drug delivery systems have also been used in transporting CBD[67]. These are mixtures of oils, surfactants and solvents that produce nano-sized droplets when they encounter an aqueous solution in the gut and their small nature means that the surface area available for drugs to be dissolved and absorbed is increased[65,67]. An example of this includes the use of micelles, which are aggregate-like structures that are soluble in water. Micelles have shown a two-fold increase in first maximum activity peak when used as hosts for cannabinoid delivery via an oral aqueous formulation[68]. Most of these systems have been explored in epilepsy or pain and further studies into their uses in cancer treatment are warranted. Additionally, CBD soft gelatin capsules have been developed as an improved formulation, and, in combination with high fat/calorie foods, may increase the rate and degree of absorption[65,69].
CBD can be administered through a transdermal route for systemic effects or topically for localised effects in the skin [70]. These forms of administration have the benefit of bypassing the first-pass metabolism effect, which results in increased drug bioavailability and continuous release over a long period of time, with less adverse effects even at higher drug concentrations[70]. Many clinical studies have investigated this method for use in fibromyalgia, epilepsy, developmental and epileptic encephalopathy, fragile X syndrome, osteoarthritis, and even ocular ailments; however, in cancer and cancer-related pain, there remains a lack of investigation[71].
The use of nanotechnology to improve and enhance efficacy of CBD delivery in vitro and in vivo has shown promise; features include enhanced encapsulation efficiency, the presence of surface functional groups that can be modified to improve stability and controlled stimuli drug release[72]. Many studies have described utilisation of this strategy for delivery of CBD and ECS components as ‘nanotherapeutics’[73]. In a syngeneic mouse model of triple-negative breast cancer, a nanomicellar formulation carrying a synthetic cannabinoid reduced tumour growth and psychoactive side effects[74]. In human glioblastoma cell lines, a lipid nanocapsule (NLC) containing CBD proved to be effective for prolonged release[75]. The blood–brain barrier (BBB) limits drug penetration into the brain; however, some studies have reported nose-to-brain administration shows a promising increased drug concentration in the brain[76]. Cannabinoids have also been loaded to poly lactic-co-glycolic acid (PLGA) nanoparticles and have shown reduced rates of tumour burden in an in ovo model of ovarian cancer[77].
CBD is highly unlikely to be used as a monotherapy for cancer treatment, but combination with chemotherapy has seen promising data in cell and mouse models of cancers; therefore, nano delivery should be considered as a tool for this synergistic therapy owing to its control of drug release reducing immunogenicity. An additional benefit of this system is the ability for conjugation of the object to the microenvironment and receptor-specific macromolecules, such as peptides, proteins and aptamers[78].
Efficacy of CBD in management of cancer and chemotherapy side effects
CBD’s non-psychoactive nature has made it an attractive target to consider for its use in cancer patients to improve their quality-of-life factors, including chemotherapy-induced pain, nausea, appetite, sleeping and anxiety[79]. Currently, the majority of data from randomised controlled trials (RCTs) have come from CBD use in epilepsy, childhood seizure disorders and chronic pain[80,81]. There is a lack of published RCT data for CBD in the palliative setting; however, some trials are underway and described below. An up-to-date clinical trials summary is presented in supplementary Table 3.
Cancer and chemotherapy-induced pain is the most studied palliative symptom and there is strong anecdotal evidence of benefit; however, supporting data from clinical trials is limited. The mechanisms that have been suggested for the analgesic effects of CBD derive from mouse models of neuropathic and taxol-induced pain[82,83]. Many studies focus on THC and THC + CBD combination rather than CBD as a single agent, which limits understanding of CBD in cancer related-pain management. For example, a randomised, double-blind, crossover study investigating a topical CBD 250mg dose up to 4 times per day in neuropathic pain reported a significant decrease in intense, sharp, cold, and itchy feelings on the Neuropathic Pain Scale (NPS), in comparison to placebo[84]. This indicates a possible role for CBD in neuropathic pain; however, the study size was small and included several aetiologies, making it difficult to transfer relevance to cancer-related neuropathic pain. In another small cross-sectional study analysing THC and CBD use in outpatient palliative care, the authors report 14 out of 58 patients taking CBD felt improvements in their pain[85]. A study of 108 patients with cancer taking medication that was THC dominant (n=52), CBD dominant (n=19) and a mixture of the two (n=33) were compared over 1 month and showed no differences between the 3 groups in pain intensity[86]. The use of paclitaxel can cause chemotherapy-induced neuropathic pain. A study found that CBD in vivo could prevent paclitaxel-induced neurotoxicity, possibly through the 5-HT1A receptor[83].
Anxiety and depression can co-exist in patients with cancer. Evidence over the years has shown CBD can reduce these symptoms. In a recent clinical trial, 14 patients with moderate-to-severe anxiety were treated for 4 weeks with a high-CBD containing sublingual solution (9.97mg/mL CBD, 0.23mg/mL THC) three times daily. Patients were aware that they were receiving CBD. The results showed a significant reduction in anxiety and improvements to mood, sleep, quality of life and cognition[87]. Although promising results were obtained, this data needs to be interpreted with caution — for example, the small cohort of patients consisted primarily of white women with above average IQ, which limits generalising, as gender and age can predominate anxiety[88]. Research has shown that sex differences significantly impact anxiolytic effects of THC, although for CBD this remains unclear and warrants the need for further investigation[89,90]. In addition, cytochrome P450 (CYP450) enzymes, which are involved in slowing drug metabolism and increasing drug effect, are also impacted by age, ethnicity and gender, which could affect metabolism of CBD[91]. CBD is also known to interact with CYP enzymes, which could cause interactions with other medications and side effects; therefore, more trials are needed to evaluate CBD’s efficacy in managing anxiety, with consideration of wider representation of patient cohort, age, sex, and potential drug–drug interactions.
A common and distressing symptom of advanced cancer is cachexia, which involves muscle wasting, owing to decreased appetite, and can cause anorexia. Reports have shown that changes to inflammatory conditions produced by the tumour and host cause an imbalance of pro (TNF-a, IL-1, IL-6, interferon g and NF-kß) and anti-inflammatory cytokines, which play a role in maintaining adipocytes, cells, neurons and bone marrow[92]. Studies have focused on use of THC + CBD or THC alone in cachexia rather than CBD alone[93–95]. One study reports a significant improvement in pre-meal appetite and a higher intake of calories consumed compared with placebo[94]. A systematic review, which analysed five studies, encompassing 934 participants taking THC alone and THC + CBD combination, found cannabis increased appetite but decreased quality of life. A possible explanation for this may be that, as CBD has anti-inflammatory properties, it may be playing a part in trying to regulate pro- and anti-inflammatory cytokines, while THC induces appetite through CB1 receptors[96,97]. With limited data available, future trials are needed to determine CBD’s role in modulating cachexia.
The ECS is known to regulate sleep; therefore, CBD and cannabis components may act as a potential therapy to aid with poor sleep, which patients with cancer can often experience. Observational studies have reported the effectiveness of CBD for improving sleep; however, RCT data is lacking[98]. Chemotherapy-induced nausea and vomiting (CINV) are common in oncology patients and cause major distress to their quality of life. CBD has shown promising effects in alleviating CINV. A randomised, double-blinded, placebo-controlled phase II trial aimed to evaluate the potential of a CBD: THC cannabis extract in preventing refractory CINV. The trial was carried out with a patient sample that experienced moderate-to-high CINV despite guideline-consistent anti-emetic prophylaxis. A total of 81 patients were randomised and given a preparation of oral CBD:THC capsules or a placebo. The researchers found that chemotherapy administration and a 2.5mg CBD/2.5mg THC dose three times daily was associated with less nausea and vomiting when compared to placebo. Overall, 31% of patients experienced sedation, dizziness and disorientation, but 83% of participants preferred cannabis to placebo and no serious adverse events were attributed to the treatment[99].
Does CBD usage affect potency of classic chemotherapy?
Evidence so far for the anticancer effects of CBD has included cytotoxic, anti-proliferative, apoptotic, autophagic and inhibiting metastasis pre-clinically. Some studies have additionally reported synergistic effects of CBD with chemo- and radiotherapy treatments. However, the mechanism for this synergy remains unclear and poses the question: does CBD usage affect the potency of these conventional anti-cancer therapies?
CBD is known to interfere and inhibit CYP450 enzymes[100]. A review by Buchtova et al. of chemotherapy drugs where CBD has reported effects separated them into the following groups: antimetabolites (small molecules mimicking standard nucleotides in DNA meaning they can synthesise DNA), alkylating agents (electron-rich atoms that can disrupt DNA function by transfer of alkyl groups), microtubule-targeting agents (bind to cytoskeletal components and can disrupt motility of the cell), anthracyclines (cause DNA damage), proteotoxic stress-inducing agents (damage protein turnover, activates unfolded protein response) and topoisomerase inhibitors (involved in DNA replication and repair damage)[101]. CBD has also been reported to have both direct and indirect antioxidant effects, in addition to reducing ROS levels and pro-inflammatory cytokines, thus reducing inflammation, which could be beneficial in reducing inflammatory responses to chemotherapy drugs[102,103].
In a study analysing CBD’s combination with paclitaxel in ovarian cancer cells, antiproliferative effects were reportedly increased with this combination and did not hinder the cytotoxic effect of chemotherapy[77]. Another report examining CBD in breast cancer cells showed a positive effect of CBD in protecting against paclitaxel-induced neurotoxicity, which was mediated partly by the 5-HT1A receptor system[83]. CBD did not attenuate paclitaxel-induced inhibition of cancer cells viability[83]. In a cell line model of glioblastoma, CBD increased the sensitivity to chemotherapy agents temozolomide (TMZ), doxorubicin, cisplatin and carmustine[104]. Clinical trials have been limited in studying the combination of CBD and chemo/radiotherapies. However, a recent two-part randomised, double-blind, placebo-controlled study by Twelves et al. of 1:1 CBD/THC + TMZ was performed in recurrent GBM patients. The authors reported that median survival was improved with the CBD/THC group when compared to placebo (550 vs. 369 days) and concluded that 1:1 CBD:THC offers some efficacy and is safe and feasible when used as an adjunct to dose-intense TMZ[105].
Overall, the benefits of CBD in combination with therapeutic agents may be explained by its influence on transporter/receptor systems. TRPV are ion channels involved in sensation of heat and pain (nociception) and stimulated by CBD[106]. A report has shown that CBD can activate TRPV2, which causes an increase in influx and retention of certain chemotherapy drugs, including TMZ and cisplatin[107]. P-glycoprotein (p-gp) confers resistance by prevention of sufficient accumulation of anticancer drugs, avoiding their cytotoxic and apoptotic effects, and is another target of CBD’s anti-tumour effects[108]. CBD has been shown to inhibit p-gp, which leads to increased accumulated levels of doxorubicin and vinblastine in cells overexpressing p-gp[109]. The multidrug resistance protein 1 (MRP1), encoded by ABCC1 and breast cancer resistance protein (BCRP)/ATP-binding cassette subfamily G member 2 (ABCG2), is an ATP-binding cassette (ABC) transporter, identified in multidrug resistance[110]. CBD can re-sensitise overexpressing cell lines to the cytotoxic effects of ABCG2 substrates, mitoxantrone and topotecan[110]. G protein-coupled receptors (GPCRs), such as GPR55, have been shown to regulate pancreatic cancer growth. In a study by Ferro et al., inhibition of GPR55 via CBD, which acts as an antagonist to this receptor, was able to reduce pancreatic ductal adenocarcinoma growth in cells[16]. In a mouse model, the administration of CBD with gemcitabine increased survival by three times when compared to gemcitabine alone[16]. Additionally, the authors found gemcitabine-resistant markers, such as ribonucleotide reductase catalytic subunit M1 (RRM1), was also reduced after CBD treatment in cells[16]. This could be leveraged for gemcitabine-resistant tumours, where CBD therapy as an adjunct with gemcitabine would allow efficient metabolism of gemcitabine in cancer cells.
Future perspective
CBD and other cannabinoids have shown promising results in pre-clinical cancer studies as cytotoxic agents, in addition to the palliative setting, as they are not associated with the adverse effects that many drugs exhibit[7]. CBD is likely to be administered in combination with chemotherapy drugs and possibly epigenetic and immunotherapy treatments, making it probable that the future of CBD will steer in the direction of adjunct therapy[111]. Given the evidence so far, CBD as an adjunct has shown leverage of standard chemotherapy agents in aiding the reversal of chemoresistance, promoting wound healing and helping to alleviate the toxic side effects, while improving quality of life of oncology patients. These notable antiemetic, anxiolytic and analgesic properties therefore make it fundamental to understand CBD as an immunomodulator to help characterise which immunotherapy and chemotherapy combinations will have synergistic efficacy[1].
Clinical studies so far have been limited; however, a three-year randomised, controlled phase II trial of temozolomide with and without nabiximols (Sativex; GW Pharma; THC:CBD combination) spray in patients with recurrent glioblastoma has commenced in the UK (ARISTOCRAT)[3,112]. A major limitation in clinical trial studies that impedes the translation of this data to clinics can be grouped into the following: (i) inefficient formulations, (ii) most appropriate drug delivery method, (iii) dosage and intervals and (iv) lack of heterogenous populations analysed and duration of treatment studied[65]. Therefore, future work should focus on monitoring longer-term usage of CBD in patients with cancer, as cancer comprises a multitude of comorbidities and cellular and molecular abnormalities, particularly in the context of potential drug reactions and toxicities. Additional research that analyses the symptoms that are best treated by CBD, the dosage, and the route should also be completed in the palliative setting.
Conclusion
CBD’s main reported mechanism of action has been through dysregulation of calcium homeostasis and mitochondrial calcium overload. However, CBD’s other direct effects via TRP, 5-HT1A and indirect effects (see Figure 1) on CB1 and CB2 are interesting avenues for exploration in cancer modulation. CBD’s pharmacokinetic and pharmacodynamic information highlights the need for further clinical studies to better understand its mechanistic actions in combination with standard chemotherapy and immunotherapy drugs, while balancing this with maintaining a safe and optimal dosing effect. In summary, CBD has the potential to hinder the growth and division of cancer cells by altering MAPK/ERK signalling. CBD can impede the formation of blood vessels by VEGF signalling. CBD exhibits anti-inflammatory properties, which is significant considering the association of chronic inflammations with cancer development through changes to NF-kß signalling.
- 1Mangal N, Erridge S, Habib N, et al. Cannabinoids in the landscape of cancer. J Cancer Res Clin Oncol. 2021;147:2507–34. https://doi.org/10.1007/s00432-021-03710-7
- 2Epidyolex 100 mg/ml oral solution. Electronic medicines compendium. 2023. https://www.medicines.org.uk/emc/product/10781/smpc#gref (accessed March 2024)
- 3Sativex Oromucosal Spray. Electronic medicines compendium. 2022. https://www.medicines.org.uk/emc/product/602 (accessed March 2024)
- 4Nabilone 1mg Capsule. Electronic medicines compendium. 2021. https://www.medicines.org.uk/emc/product/12767/smpc (accessed March 2024)
- 5Villanueva-Blasco VJ, Villanueva-Silvestre V, Vázquez-Martínez A, et al. Cannabis Use in Young and Adult University Students Before and During the COVID-19 Lockdown, According to Gender and Age. Int J Ment Health Addiction. 2022. https://doi.org/10.1007/s11469-022-00991-y
- 6Zou S, Kumar U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. IJMS. 2018;19:833. https://doi.org/10.3390/ijms19030833
- 7Martinez Naya N, Kelly J, Corna G, et al. Molecular and Cellular Mechanisms of Action of Cannabidiol. Molecules. 2023;28:5980. https://doi.org/10.3390/molecules28165980
- 8Gaoni Y, Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964;86:1646–7. https://doi.org/10.1021/ja01062a046
- 9Busquets-Garcia A, Bains J, Marsicano G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacol. 2017;43:4–20. https://doi.org/10.1038/npp.2017.206
- 10Lu H-C, Mackie K. An Introduction to the Endogenous Cannabinoid System. Biological Psychiatry. 2016;79:516–25. https://doi.org/10.1016/j.biopsych.2015.07.028
- 11Console‐Bram L, Brailoiu E, Brailoiu GC, et al. Activation of <scp>GPR</scp>18 by cannabinoid compounds: a tale of biased agonism. British J Pharmacology. 2014;171:3908–17. https://doi.org/10.1111/bph.12746
- 12Morales P, Reggio PH, Jagerovic N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Front. Pharmacol. 2017;8. https://doi.org/10.3389/fphar.2017.00422
- 13Brown KJ, Laun AS, Song Z-H. Cannabidiol, a novel inverse agonist for GPR12. Biochemical and Biophysical Research Communications. 2017;493:451–4. https://doi.org/10.1016/j.bbrc.2017.09.001
- 14Soderstrom K, Soliman E, Van Dross R. Cannabinoids Modulate Neuronal Activity and Cancer by CB1 and CB2 Receptor-Independent Mechanisms. Front. Pharmacol. 2017;8. https://doi.org/10.3389/fphar.2017.00720
- 15Laun AS, Shrader SH, Brown KJ, et al. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol Sin. 2018;40:300–8. https://doi.org/10.1038/s41401-018-0031-9
- 16Ferro R, Adamska A, Lattanzio R, et al. GPR55 signalling promotes proliferation of pancreatic cancer cells and tumour growth in mice, and its inhibition increases effects of gemcitabine. Oncogene. 2018;37:6368–82. https://doi.org/10.1038/s41388-018-0390-1
- 17Guerrero-Alba R, Barragán-Iglesias P, González-Hernández A, et al. Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol. 2019;9. https://doi.org/10.3389/fphar.2018.01496
- 18Moreno E, Cavic M, Krivokuca A, et al. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front. Pharmacol. 2019;10. https://doi.org/10.3389/fphar.2019.00339
- 19Laezza C, D’Alessandro A, Paladino S, et al. Anandamide inhibits the Wnt/β-catenin signalling pathway in human breast cancer MDA MB 231 cells. European Journal of Cancer. 2012;48:3112–22. https://doi.org/10.1016/j.ejca.2012.02.062
- 20Velasco G, Sánchez C, Guzmán M. Anticancer Mechanisms of Cannabinoids. Current Oncology. 2016;23:23–32. https://doi.org/10.3747/co.23.3080
- 21Velasco G, Sánchez C, Guzmán M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer. 2012;12:436–44. https://doi.org/10.1038/nrc3247
- 22CALVARUSO G, PELLERITO O, NOTARO A, et al. Cannabinoid-associated cell death mechanisms in tumor models. International Journal of Oncology. 2012;41:407–13. https://doi.org/10.3892/ijo.2012.1476
- 23Mahmoud AM, Kostrzewa M, Marolda V, et al. Cannabidiol alters mitochondrial bioenergetics via VDAC1 and triggers cell death in hormone-refractory prostate cancer. Pharmacological Research. 2023;189:106683. https://doi.org/10.1016/j.phrs.2023.106683
- 24Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. https://doi.org/10.1016/s0092-8674(00)81683-9
- 25Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–74. https://doi.org/10.1016/j.cell.2011.02.013
- 26Olivas-Aguirre M, Torres-López L, Valle-Reyes JS, et al. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019;10. https://doi.org/10.1038/s41419-019-2024-0
- 27Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Proliferation. 2003;36:131–49. https://doi.org/10.1046/j.1365-2184.2003.00266.x
- 28Jeong S, Yun HK, Jeong YA, et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Letters. 2019;447:12–23. https://doi.org/10.1016/j.canlet.2019.01.011
- 29Zhang X, Qin Y, Pan Z, et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules. 2019;9:302. https://doi.org/10.3390/biom9080302
- 30Pagano C, Navarra G, Coppola L, et al. Cannabinoids: Therapeutic Use in Clinical Practice. IJMS. 2022;23:3344. https://doi.org/10.3390/ijms23063344
- 31Singer E, Judkins J, Salomonis N, et al. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601–e1601. https://doi.org/10.1038/cddis.2014.566
- 32McKallip RJ, Nagarkatti M, Nagarkatti PS. Δ-9-Tetrahydrocannabinol Enhances Breast Cancer Growth and Metastasis by Suppression of the Antitumor Immune Response. The Journal of Immunology. 2005;174:3281–9. https://doi.org/10.4049/jimmunol.174.6.3281
- 33Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Seminars in Cell & Developmental Biology. 2007;18:716–31. https://doi.org/10.1016/j.semcdb.2007.09.003
- 34Pereira SR, Hackett B, O’Driscoll DN, et al. Cannabidiol modulation of oxidative stress and signalling. Neuronal Signaling. 2021;5. https://doi.org/10.1042/ns20200080
- 35Rimmerman N, Bradshaw HB, Kozela E, et al. Compartmentalization of endocannabinoids into lipid rafts in a microglial cell line devoid of caveolin‐1. British J Pharmacology. 2012;165:2436–49. https://doi.org/10.1111/j.1476-5381.2011.01380.x
- 36Salazar M, Carracedo A, Salanueva ÍJ, et al. TRB3 links ER stress to autophagy in cannabinoid antitumoral action. Autophagy. 2009;5:1048–9. https://doi.org/10.4161/auto.5.7.9508
- 37Salazar M, Carracedo A, Salanueva ÍJ, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 2009;119:1359–72. https://doi.org/10.1172/jci37948
- 38Braicu, Buse, Busuioc, et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers. 2019;11:1618. https://doi.org/10.3390/cancers11101618
- 39Yue J, López JM. Understanding MAPK Signaling Pathways in Apoptosis. IJMS. 2020;21:2346. https://doi.org/10.3390/ijms21072346
- 40Howlett AC. Cannabinoid Receptor Signaling. Handbook of Experimental Pharmacology. ;53–79. https://doi.org/10.1007/3-540-26573-2_2
- 41Javid FA, Phillips RM, Afshinjavid S, et al. Cannabinoid pharmacology in cancer research: A new hope for cancer patients? European Journal of Pharmacology. 2016;775:1–14. https://doi.org/10.1016/j.ejphar.2016.02.010
- 42Rascio F, Spadaccino F, Rocchetti MT, et al. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers. 2021;13:3949. https://doi.org/10.3390/cancers13163949
- 43Cudaback E, Marrs W, Moeller T, et al. The Expression Level of CB1 and CB2 Receptors Determines Their Efficacy at Inducing Apoptosis in Astrocytomas. PLoS ONE. 2010;5:e8702. https://doi.org/10.1371/journal.pone.0008702
- 44Glaviano A, Foo ASC, Lam HY, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22. https://doi.org/10.1186/s12943-023-01827-6
- 45Kinsey CG, Camolotto SA, Boespflug AM, et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25:620–7. https://doi.org/10.1038/s41591-019-0367-9
- 46Hu H, Tian M, Ding C, et al. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019;9. https://doi.org/10.3389/fimmu.2018.03083
- 47Matsumoto H, Miyazaki S, Matsuyama S, et al. Selection of autophagy or apoptosis in cells exposed to ER-stress depends on ATF4 expression pattern with or without CHOP expression. Biology Open. 2013;2:1084–90. https://doi.org/10.1242/bio.20135033
- 48Shrivastava A, Kuzontkoski PM, Groopman JE, et al. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Molecular Cancer Therapeutics. 2011;10:1161–72. https://doi.org/10.1158/1535-7163.mct-10-1100
- 49Su M, Mei Y, Sinha S. Role of the Crosstalk between Autophagy and Apoptosis in Cancer. Journal of Oncology. 2013;2013:1–14. https://doi.org/10.1155/2013/102735
- 50Wasik AM, Almestrand S, Wang X, et al. WIN55,212-2 induces cytoplasmic vacuolation in apoptosis-resistant MCL cells. Cell Death Dis. 2011;2:e225–e225. https://doi.org/10.1038/cddis.2011.106
- 51Blázquez C, González-Feria L, Álvarez L, et al. Cannabinoids Inhibit the Vascular Endothelial Growth Factor Pathway in Gliomas. Cancer Research. 2004;64:5617–23. https://doi.org/10.1158/0008-5472.can-03-3927
- 52Irrera N, Bitto A, Sant’Antonio E, et al. Pros and Cons of the Cannabinoid System in Cancer: Focus on Hematological Malignancies. Molecules. 2021;26:3866. https://doi.org/10.3390/molecules26133866
- 53Blázquez C, Salazar M, Carracedo A, et al. Cannabinoids Inhibit Glioma Cell Invasion by Down-regulating Matrix Metalloproteinase-2 Expression. Cancer Research. 2008;68:1945–52. https://doi.org/10.1158/0008-5472.can-07-5176
- 54Qamri Z, Preet A, Nasser MW, et al. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Molecular Cancer Therapeutics. 2009;8:3117–29. https://doi.org/10.1158/1535-7163.mct-09-0448
- 55Ramer R, Hinz B. Inhibition of Cancer Cell Invasion by Cannabinoids via Increased Expression of Tissue Inhibitor of Matrix Metalloproteinases-1. JNCI: Journal of the National Cancer Institute. 2008;100:59–69. https://doi.org/10.1093/jnci/djm268
- 56McAllister SD, Christian RT, Horowitz MP, et al. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Molecular Cancer Therapeutics. 2007;6:2921–7. https://doi.org/10.1158/1535-7163.mct-07-0371
- 57McAllister SD, Murase R, Christian RT, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. 2010;129:37–47. https://doi.org/10.1007/s10549-010-1177-4
- 58Kosgodage US, Mould R, Henley AB, et al. Cannabidiol (CBD) Is a Novel Inhibitor for Exosome and Microvesicle (EMV) Release in Cancer. Front. Pharmacol. 2018;9. https://doi.org/10.3389/fphar.2018.00889
- 59Kosgodage US, Uysal-Onganer P, MacLatchy A, et al. Cannabidiol Affects Extracellular Vesicle Release, miR21 and miR126, and Reduces Prohibitin Protein in Glioblastoma Multiforme Cells. Translational Oncology. 2019;12:513–22. https://doi.org/10.1016/j.tranon.2018.12.004
- 60Peng Y-T, Chen P, Ouyang R-Y, et al. Multifaceted role of prohibitin in cell survival and apoptosis. Apoptosis. 2015;20:1135–49. https://doi.org/10.1007/s10495-015-1143-z
- 61Jo MJ, Kim BG, Kim WY, et al. Cannabidiol Suppresses Angiogenesis and Stemness of Breast Cancer Cells by Downregulation of Hypoxia-Inducible Factors-1α. Cancers. 2021;13:5667. https://doi.org/10.3390/cancers13225667
- 62Surapaneni SK, Patel N, Sun L, et al. Anticancer and chemosensitization effects of cannabidiol in 2D and 3D cultures of TNBC: involvement of GADD45α, integrin-α5, -β5, -β1, and autophagy. Drug Deliv. and Transl. Res. 2022;12:2762–77. https://doi.org/10.1007/s13346-022-01137-2
- 63Emhemmed F, Zhao M, Yorulmaz S, et al. Cannabis sativa Extract Induces Apoptosis in Human Pancreatic 3D Cancer Models: Importance of Major Antioxidant Molecules Present Therein. Molecules. 2022;27:1214. https://doi.org/10.3390/molecules27041214
- 64Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Brit J Clinical Pharma. 2018;84:2477–82. https://doi.org/10.1111/bcp.13710
- 65Millar SA, Maguire RF, Yates AS, et al. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals. 2020;13:219. https://doi.org/10.3390/ph13090219
- 66Huestis MA. Human Cannabinoid Pharmacokinetics. Chemistry & Biodiversity. 2007;4:1770–804. https://doi.org/10.1002/cbdv.200790152
- 67Knaub K, Sartorius T, Dharsono T, et al. A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects. Molecules. 2019;24:2967. https://doi.org/10.3390/molecules24162967
- 68Kok LY, Bannigan P, Sanaee F, et al. Development and pharmacokinetic evaluation of a self-nanoemulsifying drug delivery system for the oral delivery of cannabidiol. European Journal of Pharmaceutical Sciences. 2022;168:106058. https://doi.org/10.1016/j.ejps.2021.106058
- 69Mozaffari K, Willette S, Lucker BF, et al. The Effects of Food on Cannabidiol Bioaccessibility. Molecules. 2021;26:3573. https://doi.org/10.3390/molecules26123573
- 70Mahmoudinoodezh H, Telukutla SR, Bhangu SK, et al. The Transdermal Delivery of Therapeutic Cannabinoids. Pharmaceutics. 2022;14:438. https://doi.org/10.3390/pharmaceutics14020438
- 71Palrasu M, Wright L, Patel M, et al. Perspectives on Challenges in Cannabis Drug Delivery Systems: Where Are We? Med Cannabis Cannabinoids. 2022;5:102–19. https://doi.org/10.1159/000525629
- 72Grifoni L, Vanti G, Donato R, et al. Promising Nanocarriers to Enhance Solubility and Bioavailability of Cannabidiol for a Plethora of Therapeutic Opportunities. Molecules. 2022;27:6070. https://doi.org/10.3390/molecules27186070
- 73Onaivi ES, Singh Chauhan BP, Sharma V. Challenges of cannabinoid delivery: how can nanomedicine help? Nanomedicine. 2020;15:2023–8. https://doi.org/10.2217/nnm-2020-0221
- 74Greish K, Mathur A, Al Zahrani R, et al. Synthetic cannabinoids nano-micelles for the management of triple negative breast cancer. Journal of Controlled Release. 2018;291:184–95. https://doi.org/10.1016/j.jconrel.2018.10.030
- 75Aparicio-Blanco J, Sebastián V, Benoit JP, et al. Lipid nanocapsules decorated and loaded with cannabidiol as targeted prolonged release carriers for glioma therapy: In vitro screening of critical parameters. European Journal of Pharmaceutics and Biopharmaceutics. 2019;134:126–37. https://doi.org/10.1016/j.ejpb.2018.11.020
- 76Lee D, Minko T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics. 2021;13:2049. https://doi.org/10.3390/pharmaceutics13122049
- 77Fraguas-Sánchez AI, Fernández-Carballido A, Simancas-Herbada R, et al. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. International Journal of Pharmaceutics. 2020;574:118916. https://doi.org/10.1016/j.ijpharm.2019.118916
- 78Yu B, Tai HC, Xue W, et al. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Molecular Membrane Biology. 2010;27:286–98. https://doi.org/10.3109/09687688.2010.521200
- 79Lord S, Hardy J, Good P. Does Cannabidiol Have a Benefit as a Supportive Care Drug in Cancer? Curr. Treat. Options in Oncol. 2022;23:514–25. https://doi.org/10.1007/s11864-021-00934-0
- 80O’Brien K. Cannabidiol (CBD) in Cancer Management. Cancers. 2022;14:885. https://doi.org/10.3390/cancers14040885
- 81Pauli CS, Conroy M, Vanden Heuvel BD, et al. Cannabidiol Drugs Clinical Trial Outcomes and Adverse Effects. Front. Pharmacol. 2020;11. https://doi.org/10.3389/fphar.2020.00063
- 82Mlost J, Bryk M, Starowicz K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. IJMS. 2020;21:8870. https://doi.org/10.3390/ijms21228870
- 83Ward SJ, McAllister SD, Kawamura R, et al. Cannabidiol inhibits paclitaxel‐induced neuropathic pain through 5‐<scp>HT1A</scp> receptors without diminishing nervous system function or chemotherapy efficacy. British J Pharmacology. 2014;171:636–45. https://doi.org/10.1111/bph.12439
- 84Xu DH, Cullen BD, Tang M, et al. The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities. CPB. 2020;21:390–402. https://doi.org/10.2174/1389201020666191202111534
- 85Highet BH, Lesser ER, Johnson PW, et al. Tetrahydrocannabinol and Cannabidiol Use in an Outpatient Palliative Medicine Population. Am J Hosp Palliat Care. 2020;37:589–93. https://doi.org/10.1177/1049909119900378
- 86Aviram J, Lewitus G, Vysotski Y, et al. Short-Term Medical Cannabis Treatment Regimens Produced Beneficial Effects among Palliative Cancer Patients. Pharmaceuticals. 2020;13:435. https://doi.org/10.3390/ph13120435
- 87Dahlgren MK, Lambros AM, Smith RT, et al. Clinical and cognitive improvement following full-spectrum, high-cannabidiol treatment for anxiety: open-label data from a two-stage, phase 2 clinical trial. Commun Med. 2022;2. https://doi.org/10.1038/s43856-022-00202-8
- 88McLean CP, Asnaani A, Litz BT, et al. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research. 2011;45:1027–35. https://doi.org/10.1016/j.jpsychires.2011.03.006
- 89Cooper ZD, Craft RM. Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacol. 2017;43:34–51. https://doi.org/10.1038/npp.2017.140
- 90Sholler DJ, Strickland JC, Spindle TR, et al. Sex differences in the acute effects of oral and vaporized cannabis among healthy adults. Addiction Biology. 2020;26. https://doi.org/10.1111/adb.12968
- 91Liu J, Scott BW, Burnham WM. Effects of cannabidiol and Δ9-tetrahydrocannabinol in the elevated plus maze in mice. Behavioural Pharmacology. 2021;33:206–12. https://doi.org/10.1097/fbp.0000000000000636
- 92Loberg RD, Bradley DA, Tomlins SA, et al. The Lethal Phenotype of Cancer: The Molecular Basis of Death Due to Malignancy. CA: A Cancer Journal for Clinicians. 2007;57:225–41. https://doi.org/10.3322/canjclin.57.4.225
- 93Bar-Sela G, Zalman D, Semenysty V, et al. The Effects of Dosage-Controlled Cannabis Capsules on Cancer-Related Cachexia and Anorexia Syndrome in Advanced Cancer Patients: Pilot Study. Integr Cancer Ther. 2019;18:153473541988149. https://doi.org/10.1177/1534735419881498
- 94Brisbois TD, de Kock IH, Watanabe SM, et al. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Annals of Oncology. 2011;22:2086–93. https://doi.org/10.1093/annonc/mdq727
- 95Strasser F, Luftner D, Possinger K, et al. Comparison of Orally Administered Cannabis Extract and Delta-9-Tetrahydrocannabinol in Treating Patients With Cancer-Related Anorexia-Cachexia Syndrome: A Multicenter, Phase III, Randomized, Double-Blind, Placebo-Controlled Clinical Trial From the Cannabis-In-Cachexia-Study-Group. JCO. 2006;24:3394–400. https://doi.org/10.1200/jco.2005.05.1847
- 96Wang Y, Wang X, Yang Y, et al. Comparison of the in vitro Anti-Inflammatory Effect of Cannabidiol to Dexamethasone. CCID. 2022;Volume 15:1959–67. https://doi.org/10.2147/ccid.s378798
- 97Hammond S, Erridge S, Mangal N, et al. The Effect of Cannabis-Based Medicine in the Treatment of Cachexia: A Systematic Review and Meta-Analysis. Cannabis and Cannabinoid Research. 2021;6:474–87. https://doi.org/10.1089/can.2021.0048
- 98Moltke J, Hindocha C. Reasons for cannabidiol use: a cross-sectional study of CBD users, focusing on self-perceived stress, anxiety, and sleep problems. J Cannabis Res. 2021;3. https://doi.org/10.1186/s42238-021-00061-5
- 99Grimison P, Mersiades A, Kirby A, et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomised, placebo-controlled, phase II crossover trial. Annals of Oncology. 2020;31:1553–60. https://doi.org/10.1016/j.annonc.2020.07.020
- 100Doohan PT, Oldfield LD, Arnold JC, et al. Cannabinoid Interactions with Cytochrome P450 Drug Metabolism: a Full-Spectrum Characterization. AAPS J. 2021;23. https://doi.org/10.1208/s12248-021-00616-7
- 101Buchtova T, Lukac D, Skrott Z, et al. Drug–Drug Interactions of Cannabidiol with Standard-of-Care Chemotherapeutics. IJMS. 2023;24:2885. https://doi.org/10.3390/ijms24032885
- 102Atalay S, Jarocka-Karpowicz I, Skrzydlewska E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants. 2019;9:21. https://doi.org/10.3390/antiox9010021
- 103Pagano C, Savarese B, Coppola L, et al. Cannabinoids in the Modulation of Oxidative Signaling. IJMS. 2023;24:2513. https://doi.org/10.3390/ijms24032513
- 104Deng L, Ng L, Ozawa T, et al. Quantitative Analyses of Synergistic Responses between Cannabidiol and DNA-Damaging Agents on the Proliferation and Viability of Glioblastoma and Neural Progenitor Cells in Culture. J Pharmacol Exp Ther. 2016;360:215–24. https://doi.org/10.1124/jpet.116.236968
- 105Twelves C, Short S, Wright S, et al. A two-part safety and exploratory efficacy randomized double-blind, placebo-controlled study of a 1:1 ratio of the cannabinoids cannabidiol and delta-9-tetrahydrocannabinol (CBD:THC) plus dose-intense temozolomide in patients with recurrent glioblastoma multiforme (GBM). JCO. 2017;35:2046–2046. https://doi.org/10.1200/jco.2017.35.15_suppl.2046
- 106Scott KA, Dalgleish AG, Liu WM. The Combination of Cannabidiol and Δ9-Tetrahydrocannabinol Enhances the Anticancer Effects of Radiation in an Orthotopic Murine Glioma Model. Molecular Cancer Therapeutics. 2014;13:2955–67. https://doi.org/10.1158/1535-7163.mct-14-0402
- 107Nabissi M, Morelli MB, Santoni M, et al. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2012;34:48–57. https://doi.org/10.1093/carcin/bgs328
- 108Zhu H-J, Wang J-S, Markowitz JS, et al. Characterization of P-glycoprotein Inhibition by Major Cannabinoids from Marijuana. J Pharmacol Exp Ther. 2006;317:850–7. https://doi.org/10.1124/jpet.105.098541
- 109Holland ML, Panetta JA, Hoskins JM, et al. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochemical Pharmacology. 2006;71:1146–54. https://doi.org/10.1016/j.bcp.2005.12.033
- 110Holland ML, Lau DTT, Allen JD, et al. The multidrug transporter ABCG2 (BCRP) is inhibited by plant‐derived cannabinoids. British J Pharmacology. 2007;152:815–24. https://doi.org/10.1038/sj.bjp.0707467
- 111Griffiths C, Aikins J, Warshal D, et al. Can Cannabidiol Affect the Efficacy of Chemotherapy and Epigenetic Treatments in Cancer? Biomolecules. 2021;11:766. https://doi.org/10.3390/biom11050766
- 112Pioneering trial of cannabinoid-based drug for brain tumours. University of Leeds. 2023. https://www.leeds.ac.uk/news-health/news/article/5292/pioneering-trial-of-cannabinoid-based-drug-for-brain-tumours (accessed March 2024)