Shutterstock.com
This content was published in 2010. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Objectives
Studying this article will help you gain a better understanding of:
- What inotropes are
- Why inotropes are used
- The role of different types of inotrope, namely catecholamines, phosphodiesterase-3 inhibitors and levosimendan
- The need for close monitoring of inotrope therapy in intensive care
Inotropes are a group of drugs that alter the contractility of the heart. Positive inotropes increase the force of contraction of the heart, whereas negative inotropes weaken it.
Despite this definition, when the term “inotrope” is used clinically, it is in reference to medicines that increase the force of contraction of the heart.
Negative inotropes (eg, some calcium channel blockers), which can be used to decrease the workload of the heart, are outside the scope of this article.
The rationale for inotrope use
Inotropes are indicated in acute conditions where there is low cardiac output (CO),such as cardiogenic shock following myocardial infarction, acute decompensated heart failure and low CO states after cardiac surgery.
Reduced CO leads to tissue hypoperfusion and subsequent hypoxia.Metabolism switches from aerobic to anaerobic, resulting in the formation of lactic acid. If left untreated, this can result in multi-organ failure and death.
Mean arterial pressure
Tissue perfusion is dependent on mean arterial blood pressure (MAP), which is the product of CO and systemic vascular resistance (SVR):
MAP = CO x SVR
Reducing CO or SVR lowers the MAP and therefore reduces tissue perfusion.
Systemic vascular resistance
SVR can be described simply as the resistance to blood flow (CO). It is often called the “afterload”.
Cardiac output
CO is dependent on the stroke volume (SV) and heart rate (HR):
CO = HR x SV
The SV depends on the SVR (afterload) and the “preload”. Preload (the degree to which ventricles are stretched before contracting) correlates with the end diastolic volume (the volume of blood in a ventricle at the end of filling).
It is important to optimise preload by correcting fluid balance before starting inotropes, since there is little point in increasing the contractility of the heart if its chambers are not filled optimally. Central venous pressure (CVP) can be used as a surrogate measure of preload.
Why are inotropes useful?
Inotropes increase CO, thereby increasing MAP and maintaining perfusion to vital organs and tissues. Inotropes increase CO by increasing both SV and HR.
In the failing heart, SV can only increase to a certain level before the cardiac muscle fibres become overstretched and CO will start to drop. This phenomenon is known as Starling’s law. Moreover, evidence suggests that long-term use of inotropes increases mortality.1,2
Mechanism of action
The main mechanism of action for most inotropes involves increasing intracellular calcium, either by increasing influx to the cell during the action potential or increasing release from the sarcoplasmic reticulum.
Choice of inotrope will depend on factors such as the patient’s underlying disease state and the clinician’s preference. There is limited evidence to suggest that one particular inotrope is better than another.1,2
Catecholamines
The most commonly used inotropes are the catecholamines; these can be endogenous (eg, adrenaline, noradrenaline)or synthetic (eg, dobutamine, isoprenaline).
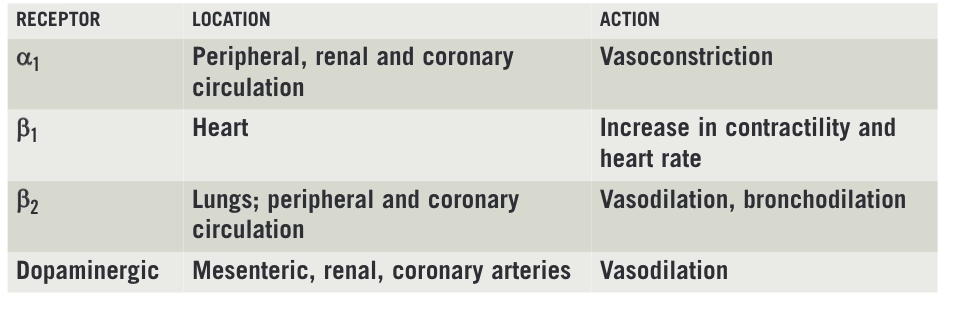
These medicines act on the sympathetic nervous system. Most commonly their cardiac effects are attributed to stimulation of alpha and beta adrenergic receptors (specifically α1, β1, and β2). However, this is a simplification: there are several subtypes of a1receptors as well as α2, β3, and various dopaminergic receptors that are involved. The location of these receptors and effects mediated via these receptors are summarised in Box 1.
The main receptor in the cardiac muscle that affects the rate and force of contraction is the β1 receptor. Binding to β1 receptors results in increased calcium entry into the cell via the opening of L-type calcium channels and release of intracellular calcium from the sarcoplasmic reticulum. More calcium is available to bind with troponin-C, thereby enhancing myocardial contractility.
Most catecholamines have a short half-life (about two minutes) and steady-state blood concentrations are reached within 10 minutes. They are therefore usually given by continuous infusion.
Box 1: Location and action of receptors
Dobutamine
Dobutamine is predominantly a β1 agonist and therefore increases cardiac contractility and heart rate. It also acts at β2 receptors causing vasodilation and decreasing afterload. Because of this vasodilation, and to ensure adequate MAPis achieved, it may be necessary to administer dobutamine in combination with a vasopressor (eg, noradrenaline).
The main side effects of dobutamine are increased heart rate, arrhythmias and raised myocardial oxygen demand. These can cause myocardial ischaemia.
Isoprenaline
Isoprenaline has a similar profile to dobutamine but tends to cause more tachycardia. It is sometimes used for bradycardic patients requiring inotropic support.
Noradrenaline
Because noradrenaline acts primarily via a1 receptors, it is usually used as a vasopressor (increasing SVR to maintain MAP) rather than an inotrope. It is often used with other inotropes, such as dobutamine, to maintain adequate perfusion, as discussed above.
Adrenaline
Adrenaline has activity at all adrenergic receptors (predominantly acting as a β agonist in low doses and an α agonist at higher doses); other more specific inotropes are often preferred over adrenaline. Adrenaline is used mainly during resuscitation after cardiac arrest (in this case it is given as a bolus). It is not recommended for use in cardiogenic shock because of metabolic side effects, including hyperlactataemia and hyperglycaemia.3
Dopamine
Dopamine is a complicated inotrope because it has dose-dependent pharmacological effects. Low-dose dopamine (2–5μg/kg/min) exerts mainly dopaminergic effects, at medium doses (5–10μg/kg/min) the β1 inotropic effects predominate and at high doses (10–20μg/kg/min) a1vasoconstriction predominates.
Phosphodiesterase-3 inhibitors
Phosphodiesterase-3 (PDE3) is an enzyme found in cardiac and smooth muscle cells.Inhibition of PDE3 increases intracellular calcium causing vasodilation and increased myocardial contractility.
The mechanism of action is independent of adrenergic receptors and therefore PDE3inhibitors are particularly useful if these receptors have become down-regulated (eg, in patients with chronic heart failure).
Milrinone is the most commonly used PDE3 inhibitor. It has a relatively long half-life (two hours) and can accumulate inpatients with renal failure.
Levosimendan
Levosimendan is a novel inotrope that sensitises troponin-C to calcium, thereby increasing the force of contraction. It also acts on potassium channels in smooth muscle to cause vasodilation. Levosimendan increases CO without increasing myocardial oxygen consumption.4
Levosimendan is administered as a continuous infusion (with or without an initial bolus dose) over 24 hours. It has a half-life of about one hour, but active metabolites mean that the inotropic effect can continue for up to five days after the infusion has finished.5
Despite levosimendan’s potentially favourable pharmacological profile, the evidence supporting its use remains controversial and it is expensive and unlicensed.
Monitoring
Inotropes are usually only used in clinical areas where patients’ haemodynamics can be monitored adequately. Continuous monitoring of MAP, CO and CVP allows haemodynamic changes to be detected and addressed rapidly.
It is essential that pharmacists in critical care understand the pharmacology of inotropes and the haemodynamic monitoring required to ensure safe practice. Pharmacists can help to identify medicines that might need to be stopped when these therapies are started (eg, beta-blockers).
For discussion
- Which inotrope is used routinely within the intensive care unit at your hospital?
- Do you think there is a role for alternative agents?Do you think that being able to describe the autonomic nervous system receptor location helps improve your understanding of inotropes in clinical practice?
References
1 Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. New England Journal of Medicine 1991;325:1468–75.
2 O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). American HeartJournal 1999;138:78–86.
3 Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. European Heart Journal 2008;29:2388–442.
4 Ukkonen H, Saraste M, Akkila J, et al. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clinical Pharmacology and Therapeutics 2000;68:522–31.
5 Kivikko M, Lehtonen L, Colucci WS. Sustained hemodynamic effects of intravenous levosimendan. Circulation 2003;107:81–6.
You might also be interested in…
Diltiazem use with anticoagulants associated with higher risk of bleeding compared with metoprolol in older people, study finds
Concerned patients ‘strongly’ advised to continue taking medication amid COVID-19 fears