
Palliative and end-of-life care
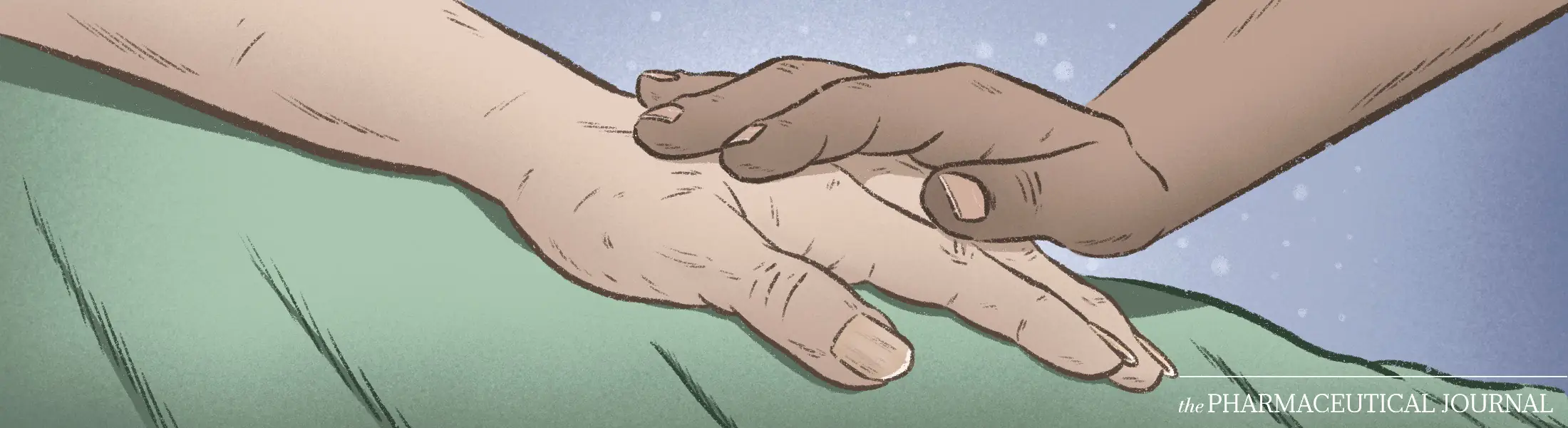
Pharmacists and pharmacy teams are vital part of the support network for patients and their families managing a terminal illness. In every healthcare setting, pharmacists can offer a wealth of information on the safe and effective use of their palliative medicines, ensuring patients can have the best quality of life in their last days.
The Pharmaceutical Journal is currently calling for ideas for blogs, comment pieces, educational support or research from all sectors of pharmacy that relate to palliative and end-of-life care. Find out more information here.
Please email us with a brief description of any initiative you are involved in that is aimed to improve access to palliative and end-of-life care or ideas for practical educational materials at: editor@pharmaceutical-journal.com
Features, opinion & podcasts


PJ view: Restore pharmacy opening hours to improve end-of-life care

How palliative care pharmacists in community practice are improving access to medicines

Controlled drug stock licensing is working against high-quality palliative care for care home patients

Effective deprescribing: getting the most from medicine
Learning

Opioid use in palliative care: selection, initiation and optimisation

Caring for palliative care patients at home: medicines management principles and considerations

Case-based learning: palliative and end-of-life care in community pharmacy
Palliative management in end-stage liver disease

Facilitating anticipatory prescribing in end-of-life care

Communicating with palliative care patients nearing the end of life, their families and carers

New priorities for palliative care
The Royal Pharmaceutical Society (RPS) and the UK’s leading end-of-life charity Marie Curie have developed eight ‘Daffodil Standards’ for palliative and end-of-life care, aimed to improve the care provided to patients approaching the end of their life through community pharmacy.
Further policy information developed by RPS Wales around palliative and end-of-life care can be found here.