Mclean / Shutterstock.com
Two of the core arms of pandemic preparedness — surveillance and vaccination — have produced ‘gold standard’ polymerase chain reaction tests for COVID-19 case monitoring and several effective vaccines for widespread population protection. But, for a time, little success was seen in antiviral research.
However, recent industry announcements suggest that antiviral achievement is starting to catch up. Furthest ahead is molnupiravir, an oral antiviral candidate from pharmaceutical giant Merck Sharp and Dohme (MSD) and partner Ridgeback Biotherapeutics. It sounds like something out of science fiction: a drug that poses as building blocks of the SARS-CoV-2 genetic code, fooling the virus into making copies of its genome that are riddled with mistakes, ultimately killing it off. However, the hope is that it will soon become reality.
Candidates from Pfizer and Roche/Atea are not far behind on the path to market, with further announcements expected in the coming weeks. But will these antivirals really make a difference, who will get them and how much will they cost? Here is what we know so far.
Could oral antivirals have a significant impact on the COVID-19 pandemic?
MSD and Ridgeback are hoping that molnupiravir — initially designed by scientists at Emory University in the United States in 2013 for Venezuelan equine encephalitis virus and named after superhero Thor’s hammer Mjölnir — is the antiviral to strike another blow to SARS-CoV-2. In October 2021, trial results announced by MSD and Ridgeback suggested that molnupiravir could halve hospitalisations and deaths from COVID-19, which led the companies to submit an emergency use authorisation application to the US Food and Drug Administration (FDA)[1].
The FDA submission was based on interim analysis of the phase II/III randomised, placebo-controlled MOVe-OUT trial, which demonstrated an approximate 50% (7.3% vs. 14.1%) reduction in risk of hospitalisation or death for individuals with mild-to-moderate COVID-19, who had at least one risk factor for severe COVID-19 and were given molnupiravir within five days of symptom onset[2]. Almost 80% of the 775 cases analysed were the prevalent gamma, mu and delta SARS-CoV-2 variants. The trial was halted early following the positive results.
In a press release, published on 1 October 2021, Robert Davis, chief executive and president of MSD, called these data “compelling”, stating “we are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic” and pledged to “do everything we can to bring molnupiravir to patients as quickly as possible”[2]. However, the data are yet to be published in a peer-reviewed journal and molnupiravir has still to be approved by regulators.
There are other antiviral candidates close behind molnupiravir in the race to market; front runners include Pfizer’s novel oral antiviral PF-07321332, which entered phase II/III trials EPIC-HR and EPIC-SR (Evaluation of Protease Inhibition for COVID-19 in High-Risk/Standard-Risk Patients) over summer 2021[3]. Speaking at Pfizer’s second-quarter sales and earnings analyst call, Mikael Dolsten, head of research and development, said he expects results shortly: “If successful, we project a potential US emergency use authorisation submission in the fourth quarter [of 2021][4].”
Another candidate at phase III trial stage is Roche and Atea’s oral antiviral AT-527, with the MORNINGSKY trial scheduled to conclude later in 2021[5].
You want something that is oral, so you can get it without having to go to the doctor, and as soon as you have symptoms, you start taking it
Janet Scott, clinical lecturer in infectious diseases at the MRC-University of Glasgow centre for virus research
If approved, these antivirals, designed in pill form, could revolutionise the way we tackle COVID-19. “You want something that is oral, so a person or a family member can go and get it without having to go to the doctor, and as soon as you have symptoms, you start taking it,” explains Janet Scott, clinical lecturer in infectious diseases at the MRC-University of Glasgow Centre for Virus Research. “And, as soon as you get a test, if it’s positive you keep taking it, if it’s negative, you stop.”
This early administration is especially significant for COVID-19. Stephen Griffin, associate professor of viral oncology at the University of Leeds, explains: “It’s about a race — and the virus gets a head start.”
Antivirals halt or reduce viral replication, but do not destroy and remove the virus directly, instead relying on the immune system to generate antibodies and clear the viral load. With SARS-CoV-2, if the immune system cannot get on top of the viral load, the infection can catalyse immune dysfunction and escalate to severe COVID-19, causing serious lung and brain complications. “If it’s got a foothold, if it’s already interfering with the immune response, if it’s already making viral particles, then you’re missing a trick,” says Griffin.
Oral antivirals provide a complementary intervention to vaccines and therapeutics; while these support the host immune response, antivirals directly target the virus. If used to prevent the virus from gaining a foothold in the first place, they could be a powerful tool to fight the pandemic.
What about preventing drug resistance?
A critical hurdle for antiviral design to overcome to be fit for real-world use is resistance — ill-considered, profligate antiviral use can rapidly negate their advantage.
“When you’ve got the virus on a huge scale like this, you’ve got a huge change engine going on,” explains Griffin. “When you start challenging [the virus] with monotherapies, you’ll see a similar thing to flu and amantadine,” he adds, referring to the first influenza antiviral that was only briefly used in the 1960s and 1970s because resistance rendered it ineffective[6].
The first challenge, therefore, is to design a drug with a high barrier for viral resistance. The current leading candidates have considered this in their design strategy.
Molnupiravir is a nucleoside analogue — a common class of antivirals that mimic the building blocks of the viral genetic material and are incorporated by the polymerase enzyme into the newly replicated RNA chain (see Figure). Although SARS-CoV-2 has a proofreader enzyme that removes genetic errors during replication, evidence suggests that the proofreader is naturally more prone to errors concerning the RNA bases molnupiravir mimics. This allows molnupiravir to work cumulatively; sustained exposure to the drug combines too many errors in the viral code for viability, resulting in viral decay[7]. Molnupiravir has proven difficult to generate resistance to under laboratory conditions, with this cumulative mechanism of action speculated to confer protection against resistance, since it does not rely on a single point of inhibition[8]. However, laboratory tests only go so far in representing real-life use — the true test will come if and when it is mass deployed.
Roche and Atea’s candidate AT-527 is another nucleoside analogue that, like molnupiravir, is incorporated into the newly replicated RNA chain. However, AT-257 can also bind to another place on the polymerase enzyme carrying out replication, in what the developers refer to as a “unique, dual mechanism that has the potential to create a high barrier to resistance” — and also avoids the proofreader enzyme.
Pfizer’s candidate, however, is a protease inhibitor, which is generally considered difficult for the virus to overcome through mutation. Protease inhibitors target the protease enzyme responsible for cutting the long polypeptide strand that is translated from the virus RNA into smaller, functional proteins. Stuck in long form, these proteins cannot carry out functions necessary for viral replication.
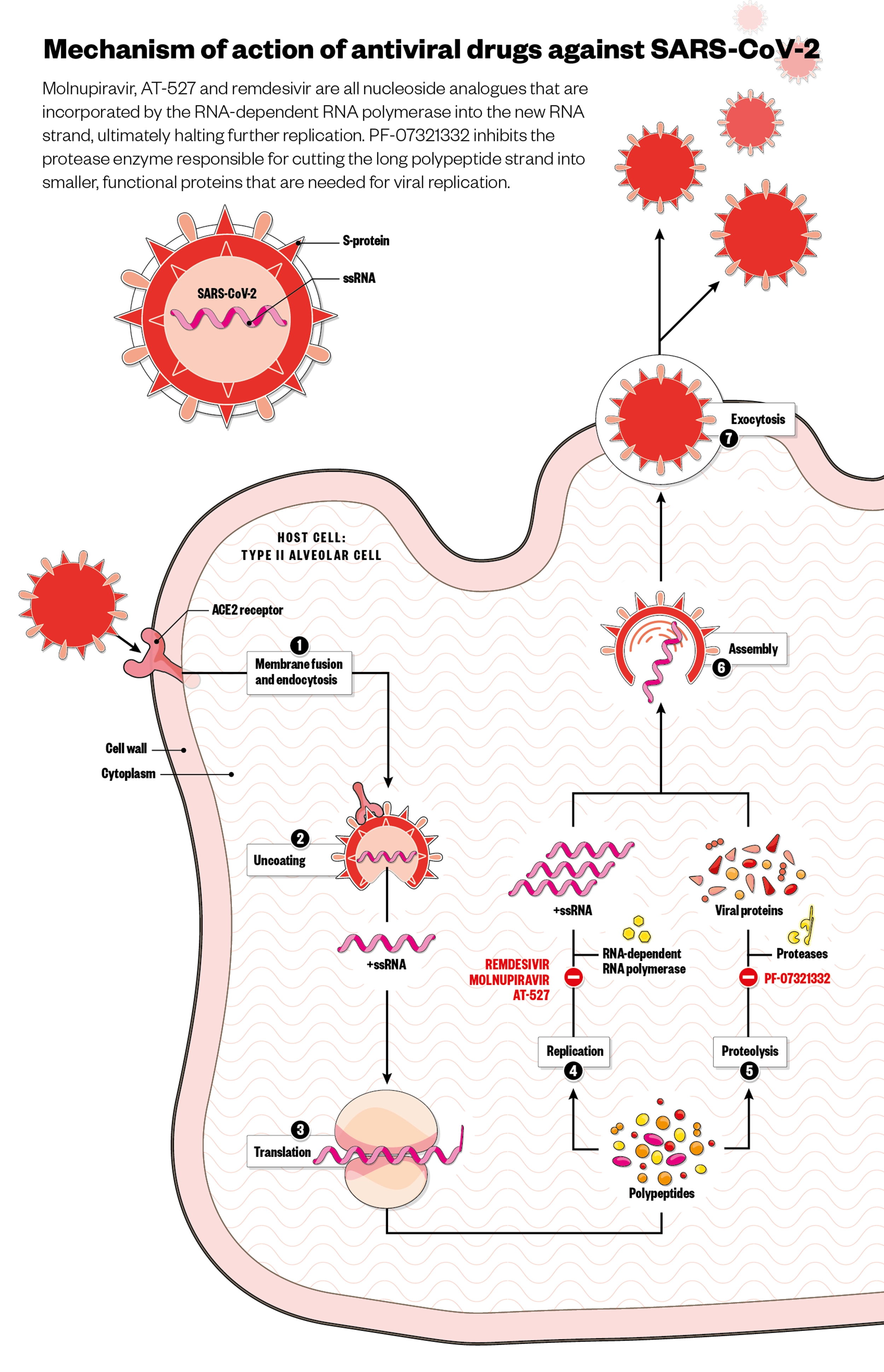
Alisdair Macdonald / Wayne Mclean
What about combination antiviral therapy, and are there other antiviral candidates being developed?
Combination therapy, common in antiviral use for other infectious diseases, such as hepatitis C and HIV, is another way of strengthening the barrier against mutation. This is because the use of antivirals with different mechanisms of action makes it harder for the virus to escape through mutation. It is something developers are exploring, such as in Pfizer’s additional investigation of PF-07321332 for use in combination with ritonavir, which is currently in phase II/III trials for patients with confirmed COVID-19 at high risk of progression to severe disease[3]. However, with limited numbers of effective antivirals, combination therapy is not a practical first approach.
Recently, virologists have been trying to utilise broad spectrum antiviral properties, “targeting enzymes that share quite significant overlap and testing against batteries of virus families to develop drugs that have activity against a multiplicity of viruses”, explains Penny Ward, Fellow of the Faculty of Pharmaceutical Medicine and visiting professor in pharmaceutical medicine at King’s College London. But while this approach provides protection against unknown, future viruses, wider-scoped mechanisms typically mean lower resistance barriers. “The difficulty is in designing this broad spectrum, very resistant-proof antiviral — it’s more difficult than designing a series of antivirals, all of which have different mechanisms,” Ward says.
Nerea Irigoyen, research lead at the University of Cambridge’s division of virology, is pursuing a broad-spectrum antiviral that hopes to accomplish just this[9]. It uses a host-targeting mechanism, something usually avoided owing to the potential for off-target effects.
The innovative thing about this is that, as we are not targeting the virus itself, we are preventing escape mutants
Nerea Irigoyen, research lead at the University of Cambridge’s division of virology
The team discovered that infection with coronaviruses activates the cellular unfolded protein response (UPR) pathway — typically activated in response to overproduction of proteins, where it works to restore the status quo. The team found SARS-CoV-2 subverts the UPR to stabilise the viral mass-production of proteins, allowing viral replication to continue at higher rates than the cell would usually cope with. Since this mechanism is only activated in stressed conditions, off-target effects of UPR inhibitors are less likely. And this host-targeting mechanism, Irigoyen points out, gives UPR inhibitors a distinct advantage when it comes to resistance. “The innovative thing about this is that, as we are not targeting the virus itself, we are preventing escape mutants.”
But there’s more: “We think that in some of the side effects of COVID-19, such as pulmonary fibrosis or even endothelial dysfunction — which is one of the major triggers of respiratory distress — the UPR can also be involved. So, we’re saying if we can reduce the UPR, we will also prevent these kind of side effects.” A UPR-inhibitor drug could bridge both antiviral and therapeutic use, providing a completely novel tool for clinicians.
How will antivirals be rolled out and how much will it cost?
“There are two ways to use antiviral medications: for the treatment of disease and symptoms to shorten the duration of illness and prevent complications like hospitalisation or death, and to prevent spread of infection to close contacts — this is called post-exposure prophylaxis,” outlines Ward.
There is a precedent for UK national antiviral strategy: in the 2009/2010 ‘swine flu’ influenza (H1N1) epidemic, the UK government implemented a mass prescription policy for the sole antiviral Tamiflu (oseltamivir; Roche).
However, Tamiflu has since been the subject of heated debate over its risk/benefit profile that, more than a decade on, has not been fully concluded[10,11]. Prescribing guidance for molnupiravir is likely to face similar hurdles.
A positive in vitro test during development indicated mutagenic potential for molnupiravir; although subsequent in vivo tests at supratherapeutic doses led the developers to conclude “molnupiravir is not considered to pose an increased risk of genotoxicity in clinical use”[12,13]. Prescribing parameters are likely to take into account possible teratogenic and genotoxic activity for at-risk populations, such as pregnant women and children.
The price of antivirals is also likely to impact how widely they can be deployed.
Treatment may need to be focused on people more likely to become more seriously ill, even if they have been vaccinated
Penny Ward, Fellow of the Faculty of Pharmaceutical Medicine and visiting professor in pharmaceutical medicine at King’s College London
If a more targeted approach is taken, “treatment may need to be focused on people more likely to become more seriously ill, even if they have been vaccinated,” continues Ward. “Or post-exposure prophylaxis aimed at preventing onwards spread of disease in higher risk communities, for example in care homes.”
The UK government announced on 20 October 2021 that it has secured 480,000 courses of molnupiravir and 250,000 courses of Pfizer’s PF–07321332/ritonavir combination and is “now working at pace on plans for deployment of the treatments, including the delivery of a national study”.
The study will allow further data to be gathered on the potential benefits these treatments bring to vaccinated patients. “Should they be approved by the medicines regulator, we could see these treatments rolled out to patients this winter, providing them with vital protection,” said Eddie Gray, chair of the UK Antiviral Taskforce, in a press release.
The Medicines and Healthcare products Regulatory Agency has confirmed that it is reviewing molnupiravir under its rolling review process, in which it assesses data as they become available from ongoing studies, before a formal application is submitted.
The US government’s advance order of 1.7 million courses of molnupiravir cost US$712 each — more than 40 times the estimated US$17.74 production cost[14]. Such an eye-watering mark-up will emphasise the need for thorough scrutiny of the data behind molnupiravir’s safety and efficacy.
How can future antiviral development be accelerated?
Several factors contributed to the delay in developing antivirals for COVID-19: funding and political will were slow to spotlight antivirals, with the UK’s Antiviral Taskforce not established until April 2021, almost a year after the Vaccine Taskforce in May 2020. And, where vaccine technology built upon an existing base of decades of research, antiviral research had much less of a head start when it came to respiratory infections[14].
Ward calls this the “vice of not preparing”.
“Disappointingly, despite the SARS epidemic in 2003 and the influenza epidemic in 2009/2010, most companies had pretty much given up development of antivirals for respiratory viruses,” Ward explains. “The mantra runs: typically, respiratory viruses are self-limiting illnesses, people recover, and usually it’s not a problem.”
It is an uncomfortable truth that acute respiratory infections were commercially unattractive. Unlike vaccines, which are used on sizeable populations, or even chronic infections, which create longer-term use dependencies, acute respiratory viruses have been subject to a general lack of investment. “Before this pandemic,” Ward continues, “apart from one or two options which had been put on the shelf during the SARS epidemic [in 2003], including remdesivir, there really weren’t any.”
Although repurposing existing drugs and redirecting drug candidates already in development for other viruses has produced promising candidates in a relatively short space of time, more investment in antivirals now may help to ward off other pandemics more quickly.
“Testing the antivirals has happened with admirable speed,” Scott concludes. “We can be proud of the way pharmaceutical science has stepped up. But it’s important to keep going, and go back far enough in the pipeline to make sure innovative solutions keep coming through.”
- 1Merck and Ridgeback Announce Submission of Emergency Use Authorization Application to the U.S. FDA for Molnupiravir, an Investigational Oral Antiviral Medicine, for the Treatment of Mild-to-Moderate COVID-19 in At Risk Adults. Merck. 2021.https://www.merck.com/news/merck-and-ridgeback-announce-submission-of-emergency-use-authorization-application-to-the-u-s-fda-for-molnupiravir-an-investigational-oral-antiviral-medicine-for-the-treatment-of-mild-to-moderate-c/ (accessed 20 Oct 2021).
- 2Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. Merck. 2021.https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/ (accessed 20 Oct 2021).
- 3COVID-19 Antiviral Efforts: Advancing Our Protease Inhibitors . Pfizer Inc. https://www.pfizer.com/science/coronavirus/antiviral-efforts (accessed 20 Oct 2021).
- 4Q2 2021 Pfizer Inc Earnings Call – Edited transcript. Pfizer Inc. 2021.https://s21.q4cdn.com/317678438/files/doc_financials/2021/q2/PFE-USQ_Transcript_2021-07-28.pdf (accessed 20 Oct 2021).
- 5Seeking to combat COVID-19 with an oral RNA viral polymerase inhibitor. Atea Pharmaceuticals. https://ateapharma.com/at-527/ (accessed 20 Oct 2021).
- 6Influenza Antiviral Drug Resistance Questions & Answers, 2021. Centers for Disease Control and Prevention. https://www.cdc.gov/flu/treatment/antiviralresistance.htm (accessed 20 Oct 2021).
- 7Holman W, Holman W, McIntosh S, et al. Accelerated first-in-human clinical trial of EIDD-2801/MK-4482 (molnupiravir), a ribonucleoside analog with potent antiviral activity against SARS-CoV-2. Trials. 2021;22. doi:10.1186/s13063-021-05538-5
- 8Agostini ML, Pruijssers AJ, Chappell JD, et al. Small-Molecule Antiviral β- d – N 4 -Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance. J Virol. 2019;93. doi:10.1128/jvi.01348-19
- 9Echavarría-Consuegra L, Cook GM, Busnadiego I, et al. Manipulation of the unfolded protein response: A pharmacological strategy against coronavirus infection. PLoS Pathog. 2021;17:e1009644. doi:10.1371/journal.ppat.1009644
- 10Jefferson T, Jones M, Doshi P, et al. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2545–g2545. doi:10.1136/bmj.g2545
- 11Tamiflu campaign. BMJ. https://www.bmj.com/tamiflu (accessed 20 Oct 2021).
- 12Painter GR, Natchus MG, Cohen O, et al. Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19. Current Opinion in Virology. 2021;50:17–22. doi:10.1016/j.coviro.2021.06.003
- 13Zhou S, Hill CS, Sarkar S, et al. β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. The Journal of Infectious Diseases. 2021. doi:10.1093/infdis/jiab247
- 14Ball P. The lightning-fast quest for COVID vaccines — and what it means for other diseases. Nature. 2020;589:16–8. doi:10.1038/d41586-020-03626-1


