Science Photo Library
The bane of a teenager’s life and among the most common skin conditions, acne vulgaris, is far from superficial. Rather, deep within the skin, four key elements are at play: androgens activate the sebaceous gland to overproduce oily sebum; dead skin cells lining the pores don’t shed properly, clogging up the hair follicle opening; the commensal bacterium Propionibacterium acnes proliferates; and immune chemicals are released causing inflammation. On the skin’s surface, blackheads and whiteheads (comedones), papules (pimples) and pus-filled spots (pustules) erupt (see Figure 1).
Acne usually starts in teenagers and can persist into adulthood, with up to half of women and 40% of men affected. The severity varies widely from person to person. Besides permanent scarring, the condition, which affects around 650 million people worldwide, can lead to anxiety and depression.
There is no cure, and available treatments have significant drawbacks, yet there have been no novel products launched over the past 10 years — innovation is long overdue.
“For many years it’s been repurposing the same old stuff,” says Adam Friedman, a dermatologist at the George Washington University School of Medicine and Health Sciences, Washington.

Courtesy of Adam Friedman
Adam Friedman, a dermatologist at the George Washington University School of Medicine and Health Sciences, Washington, says there is lots of innovation in acne drug development
Current treatments — of which there are many — aim to intervene at one or more of the four main stages (although the pathology is now known to be much more complex). Depending on severity, a mix-and-match approach is often adopted to ameliorate symptoms, and the treatments can be effective in preventing the formation of new spots and scarring.
Blackheads and whiteheads tend to be treated with topical salicylic acid or benzoyl peroxide, topical retinoids (vitamin A derivatives that act on skin and other cells) and the broad-spectrum antibiotic clindamycin. For red blemishes and pimples, oral antibiotics (especially erythromycin and tetracyclines) may be added into the mix and girls can be given combined oral contraceptive (COC) pills. For cystic acne, the oral retinoid isotretinoin (Roacutane) is often prescribed and can induce prolonged remission. “The best response is from isotretinoin — everyone improves to some degree,” says Friedman.
Salicylic acid opens pores and encourages new skin to grow, benzoyl peroxide and antibiotics kill P. acnes, while COCs suppress the activity of the sebaceous glands and reduce the formation of ovarian and adrenal androgens. The complete mechanism of action of retinoids is unknown, but topical retinoids unclog pores by decreasing the stickiness of keratinocytes inside the follicle and increasing cell turnover. Isotretinoin is highly effective at shutting down sebum production, shrinks the sebaceous gland and may also have an effect on keratinocyte shedding, says Seth Orlow, a dermatologist at New York-based academic medical centre NYU Langone Health. If they are going to work, treatments can have a measurable effect in four weeks, but many take sixteen weeks to plateau.

Courtesy of Seth Orlow
Seth Orlow, a dermatologist at New York-based academic medical centre NYU Langone Health, says that sebum inhibition has been the holy grail for dermatologists
“Although many of the medications work well if used properly, the complex combination treatment regimens required to target different aspects of acne pathophysiology lead to poor adherence, which undermines treatment success,” says Steven Feldman, a dermatologist at Wake Forest Baptist Medical Center in North Carolina. Side effects of treatments are common. Benzoyl peroxide, for example, causes redness and peeling; oestrogen hormones are unsuitable for boys; the overuse of broad-spectrum antibiotics is thought to lead to antibiotic resistance and to damage microbiota; and retinoids can cause red, sore skin that blisters and is sensitive to sunlight. The possible side effects of isotretinoin include hyperlipidaemia, abnormal liver function tests, loss of night vision, depression and suicidal thoughts. Isotretinoin is also extremely teratogenic, and so, has to be prescribed and taken with great caution.
Although many of the medications work well if used properly, the complex combination treatment regimens required to target different aspects of acne pathophysiology lead to poor adherence, which undermines treatment success
There is much room for improvement, yet no new clinically meaningful therapies have been approved in more than a decade.
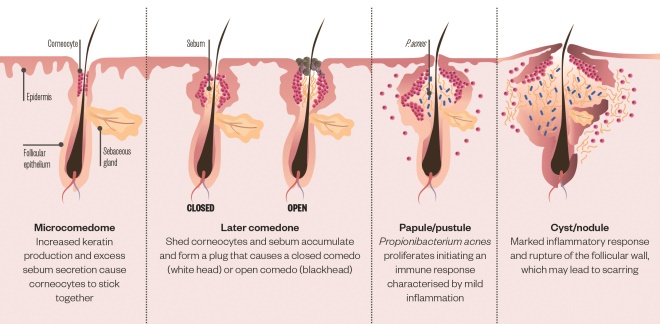
Figure 1: Acne pathogenesis
Acne is characterised by inflammation of the pilosebaceous units caused by the interplay of four key factors: excessive sebum secretion, hyperkeratinisation, colonisation of Propionibacterium acnes and inflammation
New drug targets
The good news is that scientific advances in the understanding of acne complexity are revealing new targets for development. Many receptors, cytokines, chemokines and other proinflammatory mediators are implicated, and nutrition, the skin microbiome and genetics of both the patient and the bacteria living in the follicle may also be involved[1]
. “It’s an incredibly complex phenomenon,” says Feldman.

Courtesy of Steven Feldman
Steven Feldman, a dermatologist at Wake Forest Baptist Medical Center in North Carolina, says the pathogenesis of acne is an incredibly complex phenomenon
The centrepiece of change in our understanding is what we know about the role of P. acnes, says Friedman. We have known for some time that P. acnes triggers the innate immune response by activating toll-like receptors (TLRs — gatekeepers in the immune response) on the surface of keratinocytes and immune cells such as monocytes, causing the release of inflammatory cytokines[2]
. More recently, a second gatekeeper called the NLRP3 inflammasome (a multi-protein component of the innate immune system) was also shown to be activated, leading to the secretion of proinflammatory cytokines, especially IL-1β[3]
,[4]
. This suggests that inflammation caused by P. acnes possibly could be treated by selectively targeting IL-1β or TLRs.
“We need small molecules acting upstream or downstream of IL-1β, oral or preferably topical,” says Anton Alexandroff, consultant dermatologist and spokesperson for London-based charity British Skin Foundation.
We need small molecules acting upstream or downstream of IL-1β, oral or preferably topical
It turns out that some current treatments accidentally and non-specifically act on these pathways, says Friedman. Certain retinoids, for example, have an anti-inflammatory effect by suppressing TLRs. There is also evidence that tetracycline antibiotics can inhibit the activity of neutrophils and destructive enzymes called metalloproteases, both linked to acne inflammation and scarring, says Orlow.
Approaches specifically targeting the inflammasome and IL-1β, however, are still mostly at an early stage of investigation.
Nitric oxide
A potentially promising approach is the use of nitric oxide (NO)-releasing agents, according to Alexandroff. NO has diverse functionality, including potent anti-inflammatory, antimicrobial and anti-oxidant effects[5]
.
Friedman’s group has shown that NO can inhibit multiple elements of the inflammasome complex, including IL-1β. The group is developing nanoparticles that generate NO from a nitrite salt, slowly and safely releasing NO to kill P. acnes and inhibit inflammation by acting on multiple components of the NLRP3 inflammasome[6]
. The nanoparticles are ready for testing in the clinic.
Inadvertently, [Nitrosomonas have] been washed and wiped off our super clean skin, disrupting our nitrogen cycle, and leaving our NO-mediated regulatory mechanisms out of balance
Boston-based biotech company AOBiome is taking a systems biology approach, and testing a live suspension of Nitrosomonas eutropha, Gram-negative autotrophic bacteria that oxidises ammonia and urea found on human skin to nitrite and NO. The underlying premise is that ancestral commensal Nitrosomonas historically colonised human skin as an essential component in our systemic nitrogen cycle, reducing inflammation, balancing the skin microbiome, and helping to stabilise healthy skin, explains the company’s chief medical officer Larry Weiss. These bacteria have been depleted and eliminated from modern human skin because they are incredibly sensitive to most soaps, surfactants, preservatives, and fragrances found in cosmetics and hygiene products. “Inadvertently, they’ve been washed and wiped off our super clean skin, disrupting our nitrogen cycle, and leaving our NO-mediated regulatory mechanisms out of balance,” he says. “By reintroducing Nitrosomonas to our skin, we believe we can restore this balance without returning to the life of a hunter-gatherer.” AOBiome’s spray is currently in phase IIb trials, the results of which are due by early 2018.

Courtesy of Larry Weiss
Larry Weiss, chief medical officer at Boston-based biotech company AOBiome, says the company is working on a live suspension of Nitrosomonas eutropha, Gram-negative autotrophic bacteria that oxidises ammonia and urea found on human skin to nitrite and nitic oxide
Meanwhile, researchers at University of California, San Diego (UCSD) have recently discovered another way P. acnes triggers inflammation. Short-chain fatty acids (SCFAs), produced by P. acnes when it feeds on and breaks down sebum, can leak out and trigger an inflammatory response from keratinocytes[7]
. “We found that the SCFAs inhibit an enzyme in the nucleus of cells that changes the way chromatin is folded. In skin cells, it opens up chromatin for inflammation,” explains one of the researchers Richard Gallo, a dermatologist at UCSD and chief of dermatology at the VA San Diego Healthcare System. “Inhibiting this action on the enzyme stops that inflammatory response.”
Plenty of research and development is underway, some with a shift towards targeting the early processes involved in acne development. “I am excited, there is lots of innovation,” says Friedman.
Sebum inhibitors
Skin science company Dermira is developing a novel small molecule that, if approved, will be the first to target excessive sebum production. DRM01 (olumacostat glasaretil) penetrates the skin and concentrates in the sebaceous gland, where it inhibits acetyl coenzyme-A carboxylase — an enzyme that plays an important role in the synthesis of more than 80% of sebum lipids[8]
. “We looked at animal models, and primary and established cell lines, to understand the mechanism and impact in humans,” says Eugene Bauer, co-founder of Dermira and former chair of the Department of Dermatology at the Stanford University School of Medicine. “Results so far show DRM01 has a profound effect on decreasing sebum production (e.g. 95% of triglyceride production, P. acnes’ s main food source), changing both the quality and quantity of lipid made.” He adds that preliminary toxicity data show it is “very safe and remarkably free of side effects”. Topline phase III results are expected by the middle of 2018. “Sebum inhibition has been the holy grail for dermatologists,” says Orlow.

Courtesy of Eugene Bauer
Eugene Bauer, co-founder of Dermira and former chair of the Department of Dermatology at the Stanford University School of Medicine, says the company is developing a novel small molecule that, if approved, will be the first to target excessive sebum production
Probiotics and prebiotics
There could even be a role for probiotics and prebiotics. Gallo’s team has evidence that different strains of P. acnes have different abilities to trigger inflammation. This would help explain why, although we all harbour P. acnes, not everyone develops acne. “A molecular probiotic might be able to shape the P. acnes community, minimising the ones that cause inflammation,” he says. The team is developing this idea in collaboration with MatriSys Bioscience, a skin microbiome startup in La Jolla, California.
Eric (Chun-Ming) Huang, a dermatologist at UCSD, and his team are also working on an acne probiotic project. They have recently proven that the normal skin bacterium S epidermidis in live samples of skin from acne patients can ferment sugars such as glycerol to produce SCFAs that kill P. acnes and reduce levels of proinflammatory cytokines[9]
. “A probiotic based on this finding will be a novel, effective, and safer modality for treatment of acne,” says Huang, whose team is the first in the world to establish a skin probiotic bank.
A probiotic based on this finding will be a novel, effective, and safer modality for treatment of acne
Huang’s team is also working on a vaccine to control the growth of P. acnes. The researchers have discovered that blocking Christie-Atkins-Munch-Peterson (CAMP) factor secreted by P. acnes prevents inflammation caused by P. acnes in human skin[10]
. “Using secretory CAMP factor as an antigen, the vaccine will neutralise it, reducing inflammation without killing P. acnes, which is harmless on healthy skin,” explains Huang. “This approach has the potential to result in a long-term cure of the disease.”
“The outlook for patients is very encouraging,” says Gallo. “We’ve been mired in the old approach of treating acne, using antibiotics to kill all P. acnes or using retinoids that have a number of potentially bad effects,” he explains. “Now that we understand the root cause, we can target molecular mechanisms in a safer way.”
Recent failures
Some candidates, however, have recently failed clinical trials, notably Xenon’s phase II small-molecule inhibitor of sebum production XEN801 and Novan’s SB204, an NO-releasing topical gel, which, although more effective than placebo in phase II, failed to meet one of its phase III endpoints.
“The biggest hurdle is that clinical trials require both objective and subjective testing,” says Anja Krammer, president of California-based BioPharmX, which is developing the first topical minocycline gel for acne. “The objective test counts the effect a drug has on the number of lesions. The subjective test, called the Investigator Global Assessment (IGA), relies on physicians to determine the grade improvement in each patient or the severity of those lesions,” she explains. “This understandably results in variability because each physician views a patient’s progress through his or her unique filter of experience.” She says that although a grading scale is provided during investigator training, not all physicians grade exactly the same way. “This creates challenges for developers, several of which have fallen short on their IGA scores.”
“Endpoints can be very hard to meet sometimes,” adds Friedman. “Although patients themselves may be happy, a drug will fail if it hasn’t improved enough.”
Endpoints can be very hard to meet sometimes; although patients themselves may be happy, a drug will fail if it hasn’t improved enough
However, Orlow points out that the US Food and Drug Administration is right to hold potential drugs to a high standard. “We already have lots of moderately good drugs that made the grade, we need ones that work better,” he says.
Despite the disappointments, “dermatologists and researchers are excited [about the acne pipeline],” says Alexandroff. “But Roacutane remains the benchmark (efficacy 95–97% compared with placebo).” He believes that phase III trials are needed to see if new treatments are better than Roacutane, on par with Roacutane but with better adverse-effects profile (such as teratogenicity), or at least work in patients who failed Roacutane, with the latter the most likely scenario. “So far the results are moderate — we need to keep trying.”
| Company | Candidate | Drug type | Effect | Mode of action | Development stage |
|---|---|---|---|---|---|
| AOBiome | Mother Dirt AO+ mist | Live topical bacterial spray | Anti-inflammatory, balances microbiome | Restores NO levels | Phase IIb |
| AndroScience | ASC-J9 | Topical cream | Lowers sebum | ARD enhancer | Phase III |
| Dermira | DRM01 | Topical novel small molecule | Sebum production | Acetyl coenzyme-A carboxylase inhibitor | Phase III |
| Mimetica Pty Ltd | MTC896 | Topical gel | Reduces sebum production | melanocortin-5 receptor antagonist | Phase II |
| Novan | SB204 | Topical | Antibiotic and anti-inflammatory | Releases NO, thought to inhibit upregulation of IL-1β NO | Phase III |
| Photocure | Visonac cream + red light | Photodynamic therapy | Acts on bacteria, sebaceous glands and inflammation | Photosensitisers kill cells | Phase II |
| Sebacia | Microparticles of gold and silica | Topical photoparticle therapy | Reduces sebum gland activity | Photothermal effect activated by laser | Pivotal |
| Sienna Biopharmaceuticals | Silver microparticles | Topical photoparticle therapy | Reduces sebum gland activity | Photothermal effect activated by laser | Pivotal |
| Valeant Pharmaceuticals | IDP-121 | Topical tretinoin | Unclogs pores | Retinoic acid receptor antagonist | Phase III |
| XBiotech | RA-18C3 | Subcutaneous injectable antibody | Inflammation | Blocks IL-1α (cytokine) | Phase II |
References
[1] Bhat YJ, Latief I & Hassan I. Update on etiopathogenesis and treatment of Acne. Indian J Dermatol Venereol Leprol 2017;83:298–306. doi: 10.4103/0378-6323.199581
[2] Kim J, Ochoa MT, Krutzik SR et al. Activation of toll-Like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535
[3] Qin M, Pirouz A, Kim MH et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol 2014;134:381–388. doi: 10.1038/jid.2013.309
[4] Li ZJ, Choi DK, Sohn KC et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol 2014;134:2747–2756. doi: 10.1038/jid.2014.221
[5] Schairer DO, Chouake JS & Nosanchuk JD. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012;3:271–279. doi: 10.4161/viru.20328
[6] Qin M, Landriscina A, Rosen JM et al. Nitric Oxide–releasing nanoparticles prevent Propionibacterium acnes–i nduced inflammation by both clearing the organism and inhibiting microbial stiulation of the innate immune response. J Invest Dermatol 2015;135:2723–2731. doi: 10.1038/jid.2015.277
[7] Sanford JA, Zhang LJ, Williams MR et al. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Science Immunology 2016:1; eaah4609. doi: 10.1126/sciimmunol.aah4609
[8] Hunt DW, Winters GC, Brownsey RW et al. Inhibition of sebum production with the acetyl coenzyme A carboxylase inhibitor olumacostat glasaretil. J Invest Dermatol 2017;137:1415–1423. doi: 10.1016/j.jid.2016.12.031
[9] Wang Y, Kuo S, Shu M et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol 2014;98:411–424. doi: 10.1007/s00253-013-5394-8
[10] Liu PF, Nakatsuji T, Zhu W et al. Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine 2011;29:3230–8. doi: 10.1016/j.vaccine.2011.02.036