Shutterstock
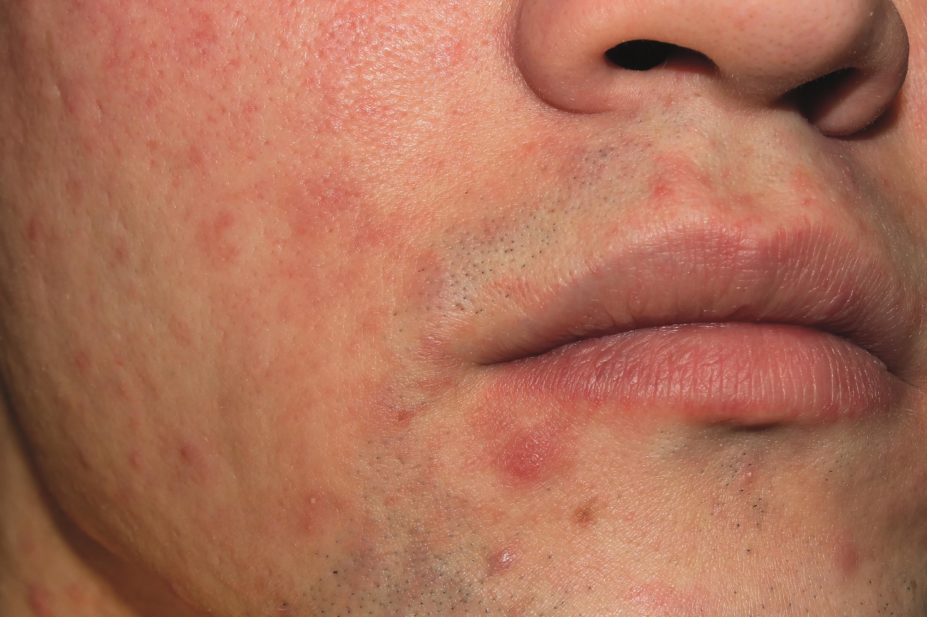
The first retinoid acne treatment to be made available over the counter (OTC) in the United States has been approved by the US Food and Drug Administration (FDA).
The topical gel adapalene, marketed as Differin Gel 0.1% by Galderma Laboratories, contains the first new active OTC ingredient for acne for more than 30 years and is approved for patients aged over 12 years.
The FDA has approved adapalene as an OTC medicine on the grounds that there is “no specific evidence” that the topical drug, if used as directed, can cause birth defects. But it does acknowledge that there have been no “adequate and well-controlled studies” involving adapalene gel and accepts that oral retinoid products can cause birth defects. It advises pregnant women, women who are thinking of becoming pregnant and those who are breast feeding to consult their doctor before using the drug.
The FDA’s decision is based on the results of five clinical trials that confirmed adapalene gel’s safety and efficacy in patients with mild to moderate acne. Results from a “maximal-use trial” showed that the drug’s absorption was limited when applied daily over a large area.
The decision coincides with a review of retinoid products launched in Europe by the European Medicines Agency (EMA), following concerns that pregnant women are still using these products to treat skin conditions despite pregnancy prevention programmes warning them about the risks the medicines pose to an unborn child.
The EMA says warnings about the risks of retinoids are inconsistent in product information across EU member states and questions how well the pregnancy prevention programmes are delivered in practice.
Community pharmacist Rod Tucker, who has an interest in dermatology and is a researcher at Robert Gordon University in Aberdeen focusing on how pharmacists can help patients with skin problems, describes the move to approve an OTC retinoid as a “major breakthrough”.
“Having a topical retinoid OTC gives pharmacists another treatment option for acne,” he says. “Retinoids affect the development of comedones (blackheads and whiteheads) so that the lesions don’t develop, basically targeting the process that leads to acne.”
Tucker, who has conducted research funded by Galderma, says it is important to distinguish the risks associated with topical compared with oral retinoids. “The risks associated with retinoids and the foetus are more to do with oral rather than topical retinoids.
“I don’t see that there is a problem with [topical] adapalene, so long as the pharmacist is sure that the woman is not pregnant or plans to become pregnant. I think most pharmacists are conscientious in that respect.”
Tucker believes giving the go-ahead to an OTC topical retinoid for the treatment of acne could lead to an OTC product combining a retinoid and a bactericidal product.
“Evidence suggests that combining a topical retinoid with benzoyl peroxide improves the treatment of acne because it targets both the formation of comedones and kills the bacteria.”
Christine Clark, a pharmacist with an interest in dermatology and former chair of the Skin Care Campaign, also applauded the FDA approval. “This is a welcome decision – particularly if it paves the way for a POM-to-P [prescription-only medicine to pharmacy medicine] move for this product in the UK.”
Clark says treatment of acne in the UK is often less than optimal. “A study presented at the British Association of Dermatologists conference last week showed that in the UK treatment of acne with systemic antibiotics continues for much longer than is recommended by evidence-based guidelines — 305 days on average vs three months[1]
.
“If antibiotic treatment is used for a long period before starting effective treatment then the risk of acne scarring can be increased.”
Clark adds that teratogenicity is a “very real problem” with oral retinoids but not with topical retinoids, but says prescribing guidelines can be “unhelpful”.
The BNF says topical retinoids are contraindicated in pregnancy and that women of childbearing age must use effective contraception. “Consumers and prescribers could find this very worrying.”
A spokesperson for the UK’s Medicines and Healthcare products Regulatory Agency, which requested the EMA to carry out its review of retinoid products, says: “We requested the EMA conduct this review, not as a safety issue but as an opportunity to ensure the warnings and risk minimisation materials provided to prescribers and patients reflect the available evidence and are proportionate to the level of harm.
“Retinoid medicines carry a level of risk associated with exposure during pregnancy and a possible risk of neuropsychiatric reactions. We want to make sure patients are getting the right information at the right time,” the spokesperson adds.
According to the EMA, oral retinoids given at the recommended therapeutic dose can harm the foetus, potentially damaging the central nervous system, and can trigger craniofacial, cardiovascular and thymic abnormalities.
Oral retinoid products have also been associated with reports of significant neuropsychiatric adverse reactions in patients, including depression, anxiety, psychotic disorder and risk of suicide, it says.
The EMA’s current advice is that oral retinoids should not be used by pregnant women. It says evidence is “less robust” against the use of topical retinoids, but it is generally recommended that these medicines should not be used during pregnancy either.
References
[1] Whitehouse HJ, Fryatt E, El-Mansori I et al. Oral antibiotics for acne: are we adopting premium use? Presented at the British Association of Dermatologists annual conference held in Birmingham on 5–7 July 2016.


