Shutterstock.com
After reading this article, you should be able to:
- Understand the importance of appropriate dosing in relation to antimicrobial resistance, patient safety and treatment outcomes;
- Define the pharmacokinetic/pharmacodynamic (PK/PD) profile of common antimicrobials;
- Understand the changes in PK/PD in patients with hepatic impairment;
- Recognise the need for dose reductions of antimicrobials in patients with hepatic impairment.
Introduction
Antimicrobial resistance (AMR) refers to the development of genetic mutations by micro-organisms (including bacteria, viruses, fungi and protozoa) that help them survive exposure to antimicrobial drugs[1]. These micro-organisms can then withstand the effects of antimicrobial drugs and continue to spread and cause infection. The rise in AMR affects the global human population and it is estimated that it already causes up to 700,000 deaths globally each year[2].
The consequences of antimicrobial resistance include:
- Difficulty in treating infections;
- Increased mortality;
- Increased hospital stays;
- Increased use of second- or third-line antimicrobial agents;
- Increased medical costs[3].
AMR is driven by the selective pressures exerted by antimicrobial exposure, either via administered treatment or secondary exposure in the environment. This is further exacerbated by overuse or inappropriate use of antimicrobial drugs[4]. Inappropriate use of antimicrobial drugs can be defined as the inappropriate drug choice and/or sub-optimal dosing, both of which are major drivers of resistance and lead to poor patient outcomes[4]. Additional information on how to evaluate the clinical appropriateness of an antimicrobial can be found here.
Antimicrobials are commonly metabolised and excreted via the hepatic and/or renal systems and, if function is impaired, antimicrobials often require dose adjustments to mitigate adverse effects without compromising on efficacy. Pharmacists can advise on dose adjustments and utilise various specialist resources to support clinical decision making.
This is the first article in a two-part series outlining the importance of antimicrobial dosing in patients with organ dysfunction. This first part will discuss antimicrobial resistance, principles guiding dose adjustments and dose adjustments in hepatic impairment.
Principles guiding dose adjustment
Overview of pharmacokinetics and pharmacodynamics
The outcome of a bacterial infection is dependent upon three factors:
- The virulence and resistance pattern of the bacterial isolate;
- The patient’s own immune response;
- The pharmacokinetics (PK) [i.e. the movement or interaction of a medicine within the body] and pharmacodynamics (PD) [i.e. the effect of the medicine on the site of action of the antibacterial used[4,5].
In microbiology, the effect of an antibacterial is quantified by the minimum inhibitory concentration (MIC), which is defined as the minimum concentration of the antibacterial that inhibits growth of a bacterial strain[4]. MIC in vitro derived laboratory values are useful for predicting in vivo treatment effects; high MIC values are associated with poor in vivo treatment outcomes and, consequently, the strain of bacteria is defined as resistant[6]. Low-level resistance (defined as two- to four-fold increases in MIC values) can be overcome with optimised antimicrobial dosing and administration. For high-level resistance, the therapeutic doses required for bactericidal or static effects are not clinically possible without direct host toxicity. For example, E. coli with amoxicillin MIC values >8mg/L are defined as resistant and are associated with treatment failure and should be avoided[6].
PK/PD profiles will vary between patient groups (e.g. children, neonates, older people, those who are critically ill and people on renal replacement therapy) and these groups will all metabolise and distribute drugs in different ways. Therefore, dosing, frequency and the administration route of the drug must be individualised for the patient, along with rate of infusions and therapeutic drug monitoring to optimise the concentration of drug within the body tissues or site of action to improve outcomes[2].
There are three PK/PD profiles that best describe the pattern/effect of antibacterial drugs; these are summarised in Table 1 and Figure 1[4,5,7]:
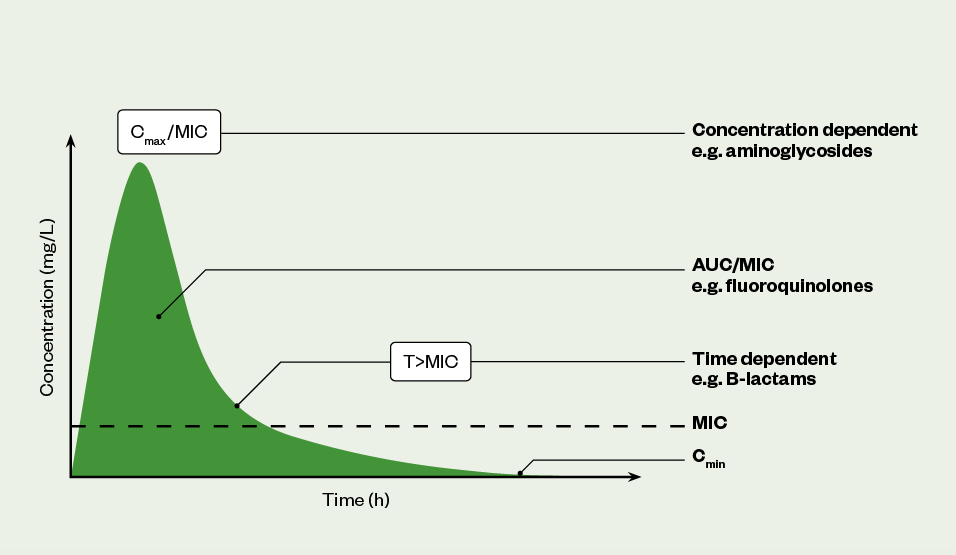
AUC: area under the curve; MIC: minimum inhibitory concentration
Dosing in hepatic dysfunction
The liver plays a central role in PK/PD of antimicrobials with direct involvement in drug bioavailability, metabolism and excretion[8–10]. Hepatic dysfunction may include synthetic liver dysfunction (loss of hepatocytes), altered volume of distribution and protein binding, altered hepatic blood flow, and reduced biliary excretion, which can all independently impact on medication PK/PD (see Figure 2)[10,11]. The aetiology underlying the patient’s hepatic dysfunction is important as it can allow for better understanding of the potential impact on a drug. Chronic viral infection (hepatitis B or C) and chronic alcohol excess are among the major causes of liver cirrhosis and can result in loss of synthetic function and decompensated hepatic dysfunction. Aging is associated with a reduction in hepatic liver blood flow and organ size, but this does not lead to routine recommendations for dose adjustments owing to the high capacity of the liver (>90% loss of function required before loss of synthetic function)[10]. The presence of ascites and other third-spaced fluid collections affect the volume of distribution and thus drug clearance of highly protein-bound antimicrobials. Acute and chronic obstructive disorders can constrict biliary excretion of common medicines, and reduce drug clearance and precipitate toxicity[8].
Unlike in renal dysfunction, there is no single marker for hepatic clearance or function to reliably guide drug dosing. Liver function tests (LFTs) can be useful in predicting hepatic function, but an understanding of the aetiology of liver insult and presence of symptoms is important for interpretation. More information on LFTs can be found here. Antimicrobial dosing may need adjusting in patients with decompensated liver disease; these patients may have LFTs within normal range but often have coagulopathy (raised prothrombin time), reduced protein synthesis (hypoalbuminaemia) and signs and symptoms of liver disease, such as ascites, oesophageal varices and encephalopathy. Transaminitis (increased ALT/AST) may be absent in decompensated liver disease owing to loss of functional hepatocytes; therefore, abnormal LFTs alone cannot be used to predict dysfunction. Presence of concurrent organ dysfunction may be present (e.g. renal dysfunction) in decompensated hepatic dysfunction to further complicate dose adjustments[8,12].
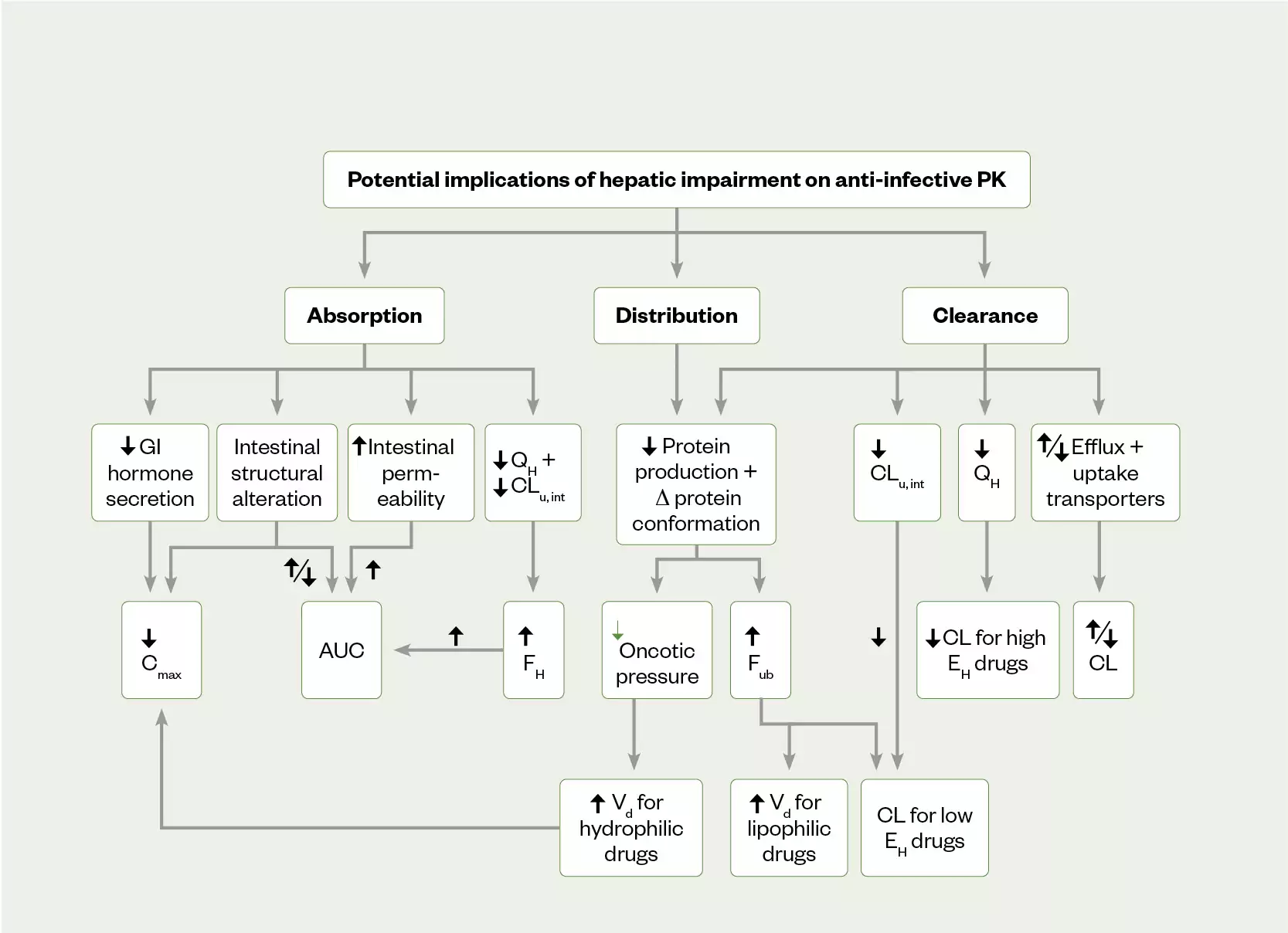
AUC: area under the curve; Cmax: maximum concentration; CLu int: intrinsic clearance of unbound drug; EH: hepatic extraction ratio; FH: hepatic bio-availability of a drug; Fub: fraction of unbound drug; GI: gastrointestinal; QH: liver blood flow; Vd: volume of distribution
Antimicrobials in hepatic dysfunction
There are limited dosing studies on commonly prescribed antimicrobials in hepatic dysfunction; therefore, explicit dosing recommendations for this cohort are not possible[13]. The Child-Pugh score system classifies mortality rates in cirrhosis patients and has been adopted for assessing severity of hepatic dysfunction using laboratory-based parameters (bilirubin, albumin and PT)[14]. Dosing recommendations are often available for Child-Pugh defined degrees of hepatic dysfunction. Medicines with high hepatic clearance, including tigecycline, metronidazole, voriconazole and caspofungin have dosing adjustment recommendations for moderate and severe hepatic dysfunction within their respective SPCs[15–18]. Highly protein-bound drugs, including the beta-lactams flucloxacillin, ertapenem and ceftriaxone, are highly susceptible to changes in albumin; hypoalbuminaemia results in increased free-unbound drug available for renal clearance and may result in sub-therapeutic serum concentrations and increased risk of treatment failures using therapeutic drug monitoring. Increasing the total daily dosing or administering parenteral doses over prolonged infusion may be useful in preventing sub-therapeutic dosing[19–23].
Where possible, using alternative antimicrobials with non-hepatic clearance or aria hypovolaemia in sepsis can occur shortly after initial septic insult. As this coincides with the introduction of antimicrobials, temporary insult is often incorrectly attributed to these agents[24].
While avoiding antimicrobials owing to the unclear nature of hepatic dysfunction or concerns about possible hepatotoxicity may be understandable, patients with hepatic dysfunction are at a much higher risk of invasive infection than those with preserved hepatic function[25]. Reduced immunity owing to hepatic dysfunction and increased risk of opportunistic infection in patients with portal vein hypertension further compound the challenges of managing this cohort, necessitating the need for pharmacy staff to optimise early antimicrobial dosing in this vulnerable patient group[25].
The management of infections in patients with renal and hepatic impairment can be challenging. The physiological changes in these disease states can alter the PK of antimicrobials, which vary on an individual basis. Standard dosing approaches can lead to AMR, treatment failure or drug toxicity. Applying individualised dosing strategies is paramount to ensure the most favourable outcomes.
The following tips can be considered when prescribing antimicrobials for patients with hepatic dysfunction:
- Avoid, where possible, therapies with known hepatotoxic properties and use the lowest effective dose for the shortest period;
- Avoid the use of therapies with narrow therapeutic windows (unless therapeutic dosing monitoring is available, e.g. voriconazole);
- Dose adjustments of antimicrobials with extensive hepatic metabolism (e.g. tigecycline or metronidazole) are required in patients with decompensated liver disease;
- Agents with non-hepatic clearance (e.g. amoxicillin) should be used where possible[8,26].
- 1Antimicrobial Resistance. Department of Health and Social Care, UK Health Security Agency, Department for Environment, Food and Rural Affairs, Veterinary Medicines Directorate. 2022.https://www.gov.uk/government/collections/antimicrobial-resistance-amr-information-and-resources (accessed Feb 2023).
- 2Tackling antimicrobial resistance 2019–2024. UK Government. 2019.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1070263/UK_AMR_5_year_national_action_plan.pdf (accessed Feb 2023).
- 3Antibiotic Resistance. World Health Organization. 2020.https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance#:~:text=Antibiotic%20resistance%20leads%20to%20higher,will%20remain%20a%20major%20threat (accessed Feb 2023).
- 4Asín-Prieto E, Rodríguez-Gascón A, Isla A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. Journal of Infection and Chemotherapy. 2015;21:319–29. doi:10.1016/j.jiac.2015.02.001
- 5Pharmacokinetics of antimicrobials. University of Dundee, British Society for Antimicrobial Chemotherapy. 2022.https://www.futurelearn.com/info/courses/antimicrobial-stewardship/0/steps/7565 (accessed Feb 2023).
- 6Mic determination of non-fastidious and fastidious organisms. European Society of Clinical Microbiology and Infectious Diseases . 2008.https://www.eucast.org/ast_of_bacteria/mic_determination (accessed Feb 2023).
- 7British National Formulary: Prescribing in renal impairment. National Institute for Health and Care Excellence. 2022.https://bnf.nice.org.uk/medicines-guidance/prescribing-in-renal-impairment/ (accessed Feb 2023).
- 8North-Lewis P, editor. Drugs and the Liver, A guide to drug handling in liver dysfunction. 1st ed. London: : Pharmaceutical Press 2008. https://www.pharmpress.com/product/9780853697107/drugs-and-the-liver (accessed Feb 2023).
- 9Diep U, Chudow M, Sunjic KM. Pharmacokinetic Changes in Liver Failure and Impact on Drug Therapy. AACN Advanced Critical Care. 2017;28:93–101. doi:10.4037/aacnacc2017948
- 10Büdingen FV, Gonzalez D, Tucker AN, et al. Relevance of liver failure for anti-infective agents: from pharmacokinetic alterations to dosage adjustments. Therapeutic Advances in Infection. 2014;2:17–42. doi:10.1177/2049936113519089
- 11Liver impairment: ensuring medicines safety. The Pharmaceutical Journal. 2015. doi:10.1211/pj.2015.20067991
- 12Hayward KL, Weersink RA. Improving Medication‐Related Outcomes in Chronic Liver Disease. Hepatol. Commun. 2020;4:1562–77. doi:10.1002/hep4.1612
- 13Weersink RA, Bouma M, Burger DM, et al. Evidence-Based Recommendations to Improve the Safe Use of Drugs in Patients with Liver Cirrhosis. Drug Saf. 2018;41:603–13. doi:10.1007/s40264-017-0635-x
- 14Guideline on the evaluation of the pharmacokinetics of medicinal. European Medicines Agency. 2005.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-impaired-hepatic-function_en.pdf (accessed Feb 2023).
- 15Tygacil 50mg powder for solution for infusion. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/200/smpc#gref (accessed Feb 2023).
- 16Metronidazole 400 mg film-coated tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/11952/smpc#gref (accessed Feb 2023).
- 17Voriconazole 200mg film-coated tablets . Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/7383/smpc#gref (accessed Feb 2023).
- 18Caspofungin 70 mg powder for concentrate for solution for infusion . Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/8956/smpc#gref (accessed Feb 2023).
- 19Ulldemolins M, Roberts JA, Wallis SC, et al. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: special emphasis on unbound pharmacokinetics. Journal of Antimicrobial Chemotherapy. 2010;65:1771–8. doi:10.1093/jac/dkq184
- 20Gavaghan V, Luu T, Adams J, et al. 602. Clinical Outcomes of Ertapenem in Patients with Hypoalbuminemia. Open Forum Infectious Diseases. 2022;9. doi:10.1093/ofid/ofac492.654
- 21Ulldemolins M, Roberts JA, Rello J, et al. The Effects of Hypoalbuminaemia on Optimizing Antibacterial Dosing in Critically Ill Patients. Clinical Pharmacokinetics. 2011;50:99–110. doi:10.2165/11539220-000000000-00000
- 22Guilhaumou R, Benaboud S, Bennis Y, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit Care. 2019;23. doi:10.1186/s13054-019-2378-9
- 23Wong G, Briscoe S, Adnan S, et al. Protein Binding of β-Lactam Antibiotics in Critically Ill Patients: Can We Successfully Predict Unbound Concentrations? Antimicrob Agents Chemother. 2013;57:6165–70. doi:10.1128/aac.00951-13
- 24Hypoxic Hepatitis: A Review and Clinical Update. JCTH. 2016. doi:10.14218/jcth.2016.00022
- 25Rolando N, Philpott-Howard J, Williams R. Bacterial and Fungal Infection in Acute Liver Failure. Semin Liver Dis. 1996;16:389–402. doi:10.1055/s-2007-1007252
- 26Zoratti C, Moretti R, Rebuzzi L, et al. Antibiotics and Liver Cirrhosis: What the Physicians Need to Know. Antibiotics. 2021;11:31. doi:10.3390/antibiotics11010031