Science Photo Library
After reading this learning article, you should be able to:
- Recommend the most appropriate antimicrobial agent;
- Determine the duration of therapy for an antimicrobial course;
- Effectively counsel patients on antimicrobial therapy;
- Identify and manage antimicrobial prescribing issues.
Antimicrobial resistance (AMR) is a growing problem and is listed in the top ten global threats facing humanity by the World Health Organization[1]. Without effective antimicrobials, major surgical procedures and chemotherapy would carry increased risk[2]. Currently, around 700,000 people die each year as a result of AMR, which is estimated to rise to 10 million deaths by 2050[3]. Several drivers are associated with this increase, including use, misuse and overuse of antimicrobials in humans, animals and agriculture; poor hygiene, water and sanitation; and a lack of research into new antimicrobials[3].
Although antimicrobial prescribing has reduced by 11% in primary care between 2013 and 2017, there has been a 7.7% increase in secondary care antimicrobial prescribing per admission during the same period[4]. Pharmacists in all sectors have a vital role in reviewing patients’ antimicrobial prescriptions to combat AMR, and play a vital part in antimicrobial stewardship (AMS) programmes by ensuring appropriate use of antimicrobials[5,6]. Pharmacists can help to prevent overuse of antimicrobials by providing advice on self-limiting conditions and also help reduce misuse by counselling patients on dosing. Pharmacists can also help prevent infections through involvement in vaccination programmes[7].
In 2015, The National Institute for Health and Care Excellence (NICE) published guidelines to establish antimicrobial stewardship systems and processes for all NHS care settings[8]. The ‘Start Smart, then Focus’ and TARGET toolkits are available for healthcare professionals to aid decision making about appropriate use of antimicrobials, with the latter including some patient education materials to promote appropriate antimicrobial use in the community[9,10]. All healthcare institutions are recommended to undertake periodic audits (at least every year) to measure compliance[8].
This article covers review of antimicrobial prescriptions, determining whether an antibiotic is appropriate, the steps to follow when inappropriate use is suspected and how to optimise antimicrobial therapy.
Indication
A 2018 review of antimicrobials prescribed in UK general practice found that up to 23% of prescriptions may be inappropriate[11]. Ensuring the presence of an infection and that antimicrobials are an appropriate treatment is therefore the first stage of an AMS intervention.
Confirming bacterial infection
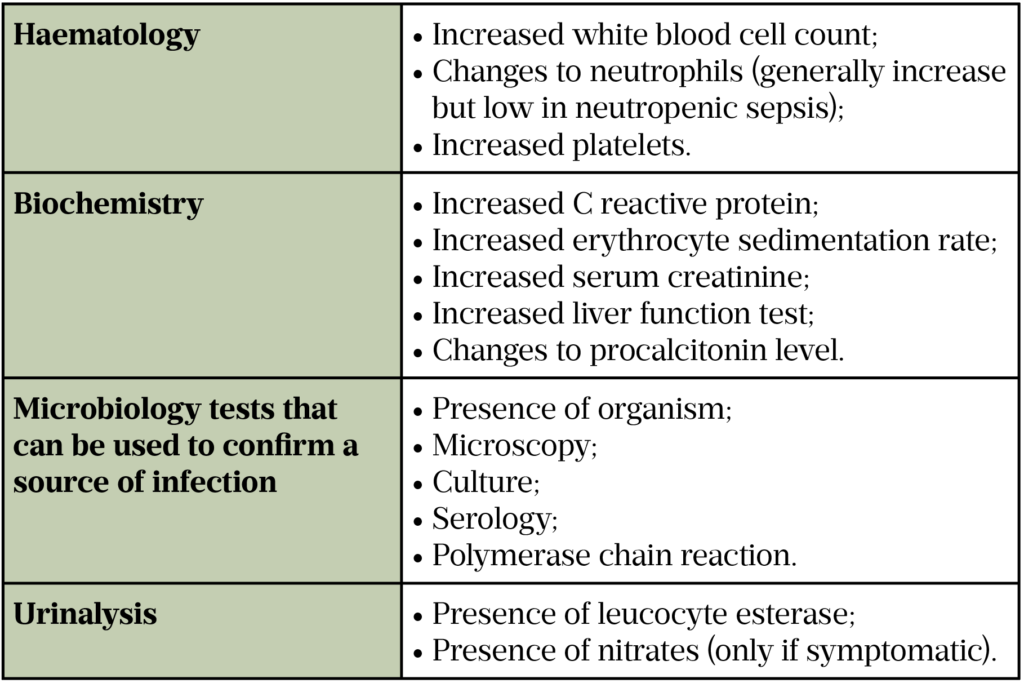
Using symptoms and laboratory markers, if available (see Table 3 and Table 4), the prescriber must consider if the cause is bacterial, viral or fungal to ensure any recommended treatment is effective[12,13].
For some infections, the illness may be self-limiting (such as cold or mild influenza), meaning an antimicrobial may be inappropriate[7]. For others, source control (physical measures used to control the foci of invasive infection and restore optimal function of the affected area), such as surgical intervention, debridement or drainage, is a necessary part of infection management[14].
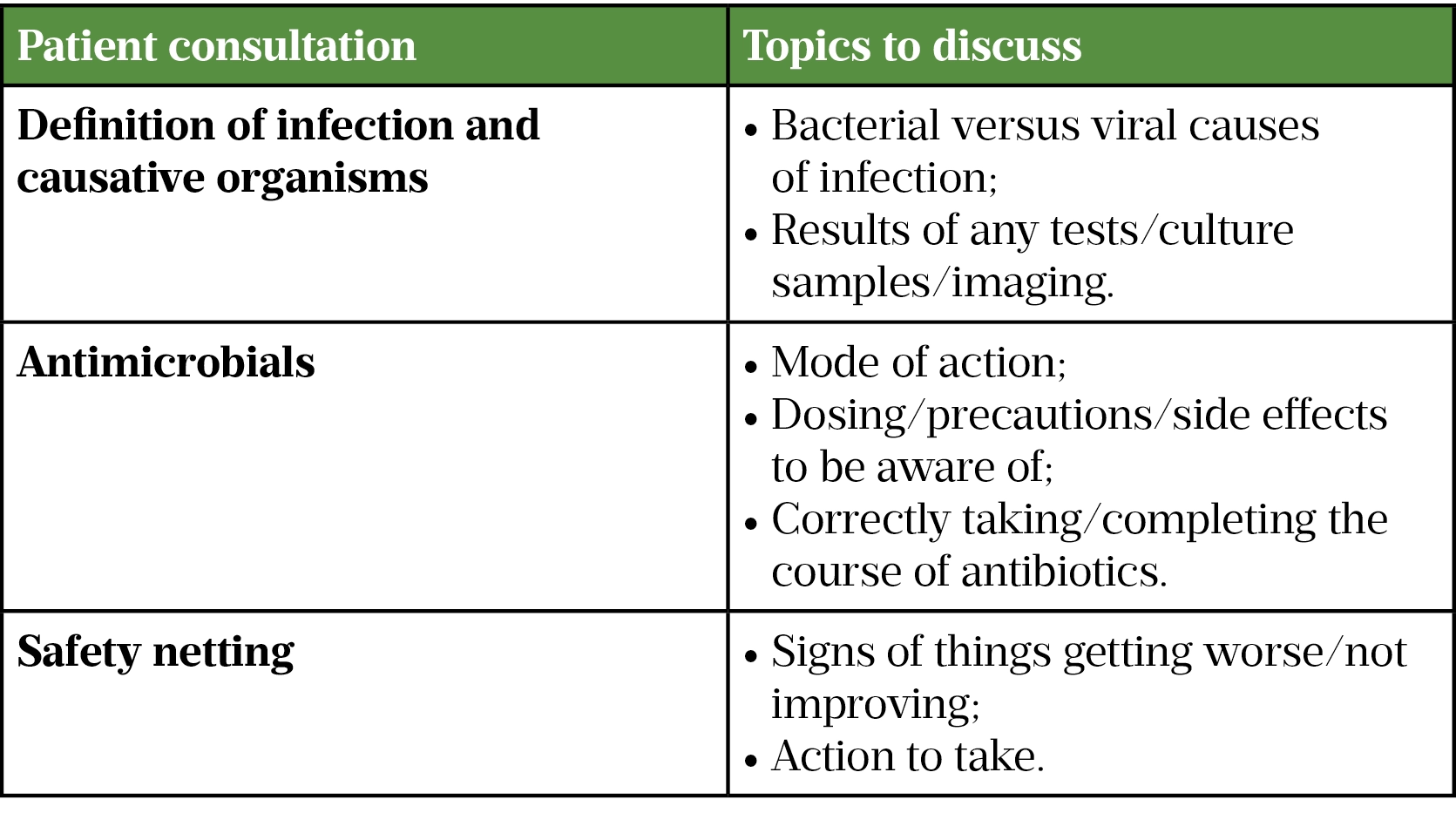
Once a clear indication for an antimicrobial is established, this should be explained to the patient (see Table 1) and communicated to other members of the clinical team through documentation in the patient’s clinical record[7–9,15]. The patient should be counselled on administration and duration of the antimicrobial course, potential side effects, signs of worsening infection and what to do if they feel unwell. This is addition to self-care and infection prevention and control measures for communicable infections to stop spread (e.g. hand hygiene)[16].
Initial empiric treatment is usually based on the most likely causative organism, therefore, microbiological samples (e.g. blood cultures, urine and sputum) should be taken prior to commencing treatment, to guide targeted switches to alternatives that are better tolerated or more effective against the pathogen involved[8,9].
Red flags, such as high temperature, hypotension, rapid pulse, high/low respiratory rate and altered mental state, can indicate sepsis (see ‘Case-based learning: recognising sepsis‘)[17]. It is important that these infections are treated promptly with appropriate antimicrobials, ideally within an hour of diagnosis[9]. In primary care settings, such as a GP clinic or community pharmacy, these symptoms should trigger urgent transfer to hospital.
Bacterial or viral infection
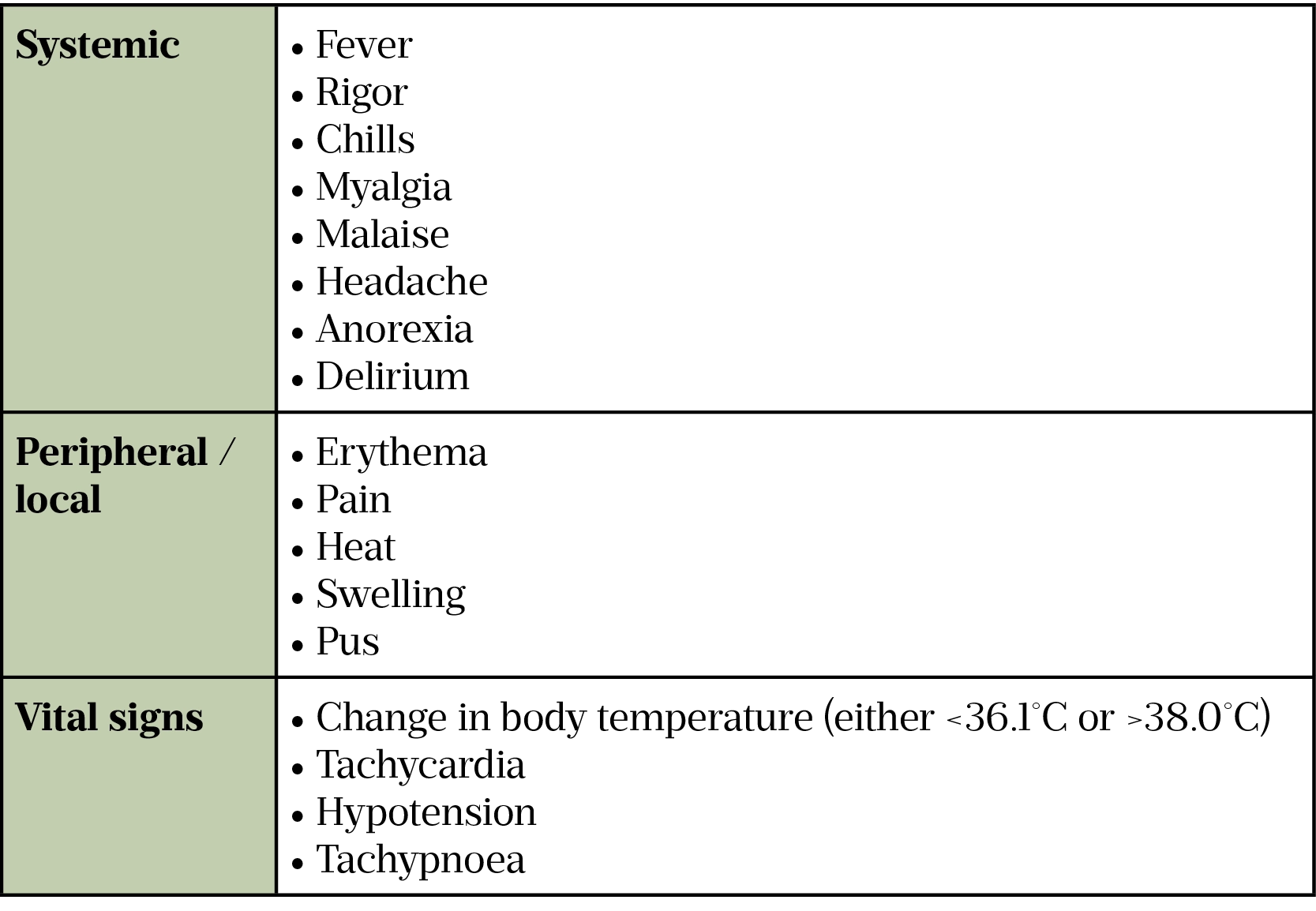
Many common infections can be caused by bacteria or viruses. Identifying the causative agent is of paramount importance to determine the best course of treatment. Signs and symptoms alone can be misleading since most viral infections present similarly to bacterial infections (e.g. viral chest infections)[18]. Inflammatory markers, such as white cell count and C-reactive protein, are often raised in both viral and bacterial infections.
To identify the causative organism, laboratory tests are ordered on blood cultures and site-specific samples such as sputum, urine or cerebrospinal fluid. Tests such as microscopic identification and polymerase chain reaction (PCR) are undertaken on these samples. A PCR test involves multiple steps of replication of RNA or DNA in the sample to enable the identification of the respective pathogen, thus confirming if the infection is of bacterial origin[13]. While these tests can provide definitive answers, they can take days to report, which delays appropriate antibiotic choice and risks antibiotic use where no bacterial pathogens are present. More rapid point-of-care testing kits are continuing to be developed and deployed for the rapid detection of various immunological markers to identify infection (e.g. influenza and COVID-19)[18].
In addition to microbiological testing, biochemical testing is used to identify the response to the infection. Procalcitonin (PCT) is a precursor of calcitonin and is naturally produced in the body by the thyroid gland to regulate calcium metabolism. High levels of PCT indicate severe bacterial infection, whereas PCT is less likely to be raised in response to viral infections, autoimmune disease or inflammatory conditions[19]. Table 2 shows ranges of PCT and the impact this may have on treatment.

A meta-analysis submitted to the FDA included 11 studies of 4,090 patients and concluded that PCT-guided patients had lower odds of antibiotic initiation (odds ratio: 0.26; 95% confidence interval: 0.13–0.52) and shorter mean antibiotic use (weighted mean difference: -2.15 days) compared with patients treated with standard care. PCT use had no impact on mortality rate or length of hospital stay[22]. A NICE review of the PCT test concluded that evidence is lacking to recommend routine use of the test in practice[20]. Further research of its impact on antibiotic use for severely ill patients and for management of COVID-19 infection is recommended[20,21].
Important considerations
In addition to treating infections, antimicrobials can be used for long-term prophylaxis (e.g. in recurrent urinary tract infections), surgical prophylaxis and as adjuvant prophylaxis (e.g. with some chemotherapy regimens)[23,24]. Primary care or specialist clinic reviews of adherence, tolerability and the ongoing need for these prophylaxis regimens are important to ensure that these are effective.
Patients at extremes of age (very young and very old), immunocompromised patients and patients with serious long-term conditions, such as chronic kidney disease or liver failure, are at higher risk of serious infections and are more likely to need antimicrobial treatment[23,24].
Selecting an antimicrobial
The choice of which antimicrobial to prescribe can often be complex and involve several factors, such as:
- Likely causative organism;
- Infection severity;
- Local resistance patterns;
- Drug interactions;
- Patient factors such as allergies, bodyweight and renal function[9,23,24].
NICE guidelines on common infections should be used as a reference by prescribers when choosing an antimicrobial[25]. The NICE guidelines are normally localised into organisational antimicrobial formularies and guidelines, which will differ slightly owing to local antibiograms (i.e. local sensitivity patterns), which may vary between areas.
There are several legitimate reasons to deviate from guidelines, including:
- Recent antimicrobial usage;
- Recent travel abroad;
- Previous multi-drug resistant infections;
- Recent hospital admissions.
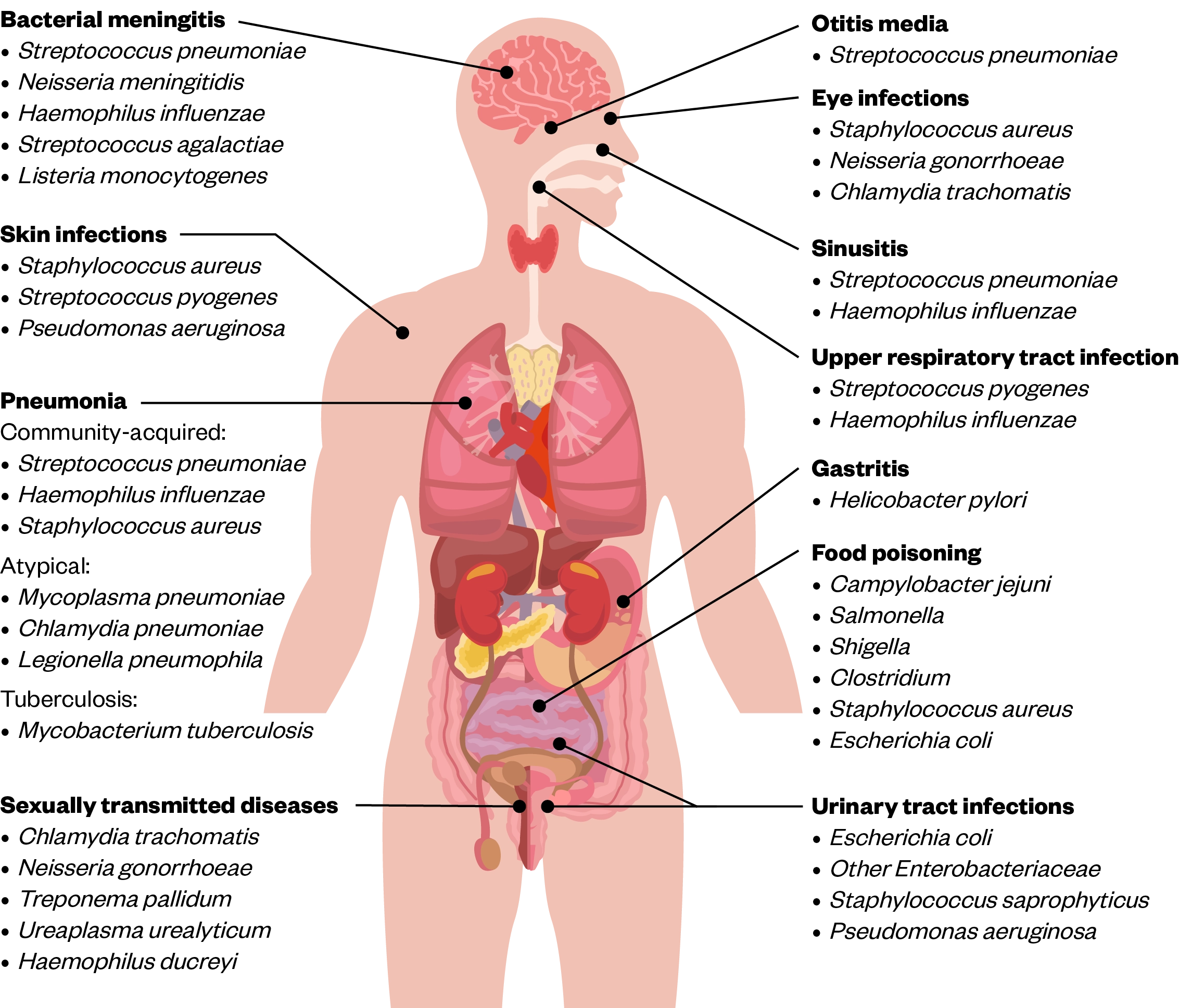
Where there are deviations from guidelines, pharmacists should establish whether the prescriber has done so appropriately. Pharmacists can do this by gaining an understanding of which pathogens are likely to cause infection in different areas within the body (see Figure). This can then guide the antimicrobials that are likely to be effective in terms of spectrum of activity and penetration[23,25].
Allergy status
Penicillins are the most prescribed antibiotic class in the UK (37.8%), followed by tetracyclines (26.4%) and macrolides (15.3%)[27]. However, around 16% of the UK population self-report an allergy to pencillin[28]. Pharmacists should establish the nature of the reaction when consulting with patients, as fewer than 10% of people who report allergy are truly allergic. Therefore, 9% of the population may be getting a less effective or less tolerable antibiotic because of erroneous allergy labelling. This is important as patients labelled as penicillin allergic are more likely to receive drugs that are broader spectrum and have the potential for more adverse reactions (including Clostridioides difficile infection) and induction of AMR[28]. For further information, see PJ articles on identification and management of penicillin allergies, penicillin allergy and resistance and diagnosing antibiotic allergies.
Contraindications and interactions
As with all reviews, pharmacists should not only assess antimicrobial appropriateness, but also consider potential drug interactions and effect on other disease states. It is essential to keep up to date with National Patient Safety Alerts and take them into consideration when checking an antimicrobial prescription (see MHRA drug safety updates).
Common contraindications include pregnancy and breastfeeding, where specialist support should be sought. Useful reference sources include Schaefer et al‘s Drugs in pregnancy and lactation or medicines information services to assess safety and appropriateness before prescribing[16,29].

In addition, caution is needed when treating patients with myasthenia gravis. It risks exacerbation from a range of antibiotics, such as aminoglycosides, clindamycin, colistin, quinolones and macrolides; hence these are contraindicated in patients with myasthenia gravis. In addition, tetracyclines, sulphonamides, vancomycin and tigecycline are cautioned and should be avoided where possible[16,30,31].
Renal function
It is important to assess patients’ renal function to ensure the correct dose and frequency of the chosen antimicrobial. Acute kidney injuries, defined by a rise in creatinine of 25 micromol/L or greater within 48 hours or a fall in urine output to less than 0.5ml/kg/hour for more than 6 hours, and chronic kidney diseases will affect the elimination of hydrophilic antimicrobials such as glycopeptides, aminoglycosides and penicillins[32].
For patients with impaired renal function, pharmacists should check whether the impairment is acute or chronic and whether they are on dialysis. Specialist sources, such as The Renal Drug Handbook, can be used to identify the most suitable dose for the patient[33]. Otherwise, regional medicines information centres can receive enquiries from community pharmacists regarding dose adjustments in renally impaired patients[16].
Antimicrobials with narrow therapeutic index, such as aminoglycosides and glycopeptides, can cause nephrotoxicity and will require therapeutic drug monitoring[16]. Pharmacists should check their local guidelines for the necessary dosing and monitoring requirements. In sepsis, reduced cardiac output and hypoperfusion can lead to pre-renal acute kidney injury, combined with fluid shifts and changes in volume of distribution[34]. However, augmented renal clearance as a result of systemic inflammatory response syndrome can actually increase renal elimination[35]. Therefore, many centres treat sepsis with standard antimicrobial dosing for the first 24–48 hours, then review the need for dosage adjustments[36,37].
Body weight
Obesity is associated with pathophysiological changes, such as altered volumes of distribution and clearance, which can lead to suboptimal dosing and treatment failure[38]. Options include increasing doses or using prolonged or continuous infusions, along with therapeutic drug monitoring to guide any dosing changes[39]. Care must be taken when checking the dose for antimicrobials with a narrow therapeutic index (e.g. glycopeptides and aminoglycosides) as using total body weight often leads to nephrotoxicity in obese patients and ideal body weight should be used instead[39].
Advice on which weight to use for dosing of a number of antibiotics can be found in a summary compiled by UK Medicines Information, the Association of Scottish Antimicrobial Pharmacists and Healthcare Improvement Scotland[39].
Duration and review
Empiric guidelines used by many NHS trusts recommend a suggested antimicrobial course length, often dependent on the site of infection, severity and causative organism[25,40]. Other patient-related factors, such as recent antimicrobial use and recurrence of infection, may dictate longer courses of antimicrobials[25]. Pharmacists should be able to explore these factors and be confident in checking that an appropriate course length has been prescribed. If the prescribed course was deemed inappropriate or further clarification is needed as to why a certain course length was selected, pharmacists should contact the prescriber as soon as practically appropriate. Pharmacists should feel empowered to discuss and challenge any inappropriate prescribing practice related to antimicrobials as they do with other medications.
After starting, an antimicrobial should be reviewed within 48–72 hours to ensure that the initial diagnosis is still valid and the chosen antimicrobial remains appropriate[8,9]. Response to the antimicrobial should also be monitored by checking patients’ temperature, inflammatory markers and general wellbeing[8]. If the antimicrobial is to continue, regular reviews every 48–72 hours should be undertaken until a final course length can be determined. The review outcome (to continue, stop or change antimicrobial) should be clearly documented in the clinical notes. In the community, the prescriber should follow up with the patient to ensure that the prescribed antimicrobial is still appropriate and the review outcome should be documented in the patient’s paper or digital health record[8,10].
If the patient is initiated on an intravenous antimicrobial, their response should be reviewed daily to ensure a timely switch to an oral alternative[9]. Intravenous administration is associated with increased risk of healthcare-associated infections such as methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia, increased discomfort for the patient and longer nursing time needed for preparation and administration[41,42]. Additionally, switching an intravenous antimicrobial to oral can facilitate patient discharge[42]. Several criteria can be checked to see if the patient is ready for an oral switch, such as the ACED criteria (see Box 1)[43].
Box 1: Consideration for intravenous to oral switch — the ACED criteria
A — Afebrile for more than 24 hours (i.e. haemodynamically stable with no signs of fever);
C — Clinically improving — improving signs and symptoms of infection and inflammatory markers trending down towards normal;
E — Eating and drinking — they have a functional GI tract with no malabsorption and there are no interactions with other medications;
D — Not suffering from certain Deep-seated/high-risk infections (such as liver abscess, osteomyelitis, septic arthritis, meningitis, endocarditis).
Source: Leeds Health Pathways [43]
When the decision is made to swap to an oral antibiotic, the following should be considered[42,44]:
- Patient’s clinical presentation (e.g. temperature, blood pressure and general wellbeing). Signs and symptoms specific to the infection should be reviewed — is there clear improvement compared with initial presentation?
- Patient should be able to absorb medications orally. An easy indication is to check if the patient is eating and drinking with no nausea or vomiting.
- Certain deep-seated infections will require prolonged courses of intravenous antimicrobials (e.g. septic arthritis and endocarditis). If in doubt, check antimicrobial guidelines or discuss with a consultant microbiologist.
Patient counselling
Pharmacists should use every opportunity to educate patients and the public about appropriate use of antimicrobials. Counselling patients to take their antimicrobials as prescribed is as important as educating them about managing minor infections without antimicrobials[6,45]. Pharmacists should take part in national campaigns, such as Antimicrobial Awareness Week and Antibiotic Guardian, to help improve public education on this vital topic[6].
References
- 1Ten Threats to Global Health. World Health Organization. 2019.https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed Jul 2021).
- 2Antimicrobial resistance. World Health Organization. 2020.https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed Jul 2021).
- 3No time to waste: securing the future from drug-resistant infections. Report to the secretary-general of the United Nations. World Health Organization. 2019.https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1 (accessed Jul 2021).
- 4NICE Impact: Antimicrobial resistance. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/media/default/about/what-we-do/into-practice/measuring-uptake/niceimpact-antimicrobial-resistance.pdf (accessed Jul 2021).
- 5Tackling Antimicrobial Resistance 2019-2024: The UK’s five-year national action plan. HM Government. 2019.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf (accessed Jul 2021).
- 6RPS launches major new campaign on antimicrobial stewardship. The Pharmaceutical Journal Published Online First: 2017. doi:10.1211/pj.2017.20203484
- 7Antimicrobial stewardship (AMS): A quick reference guide. Royal Pharmaceutical Society of Great Britain. 2017.https://www.rpharms.com/LinkClick.aspx?fileticket=iV0r2V67dV0%3d&tabid=2088&portalid=0 (accessed Jul 2021).
- 8National Institute for Health and Care Excellence. Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use (NG15). 2015.https://www.nice.org.uk/guidance/ng15 (accessed Jul 2021).
- 9Public Health England. Antimicrobial stewardship: start smart then focus. 2015.https://www.gov.uk/government/publications/antimicrobial-stewardship-start-smart-then-focus (accessed Jul 2021).
- 10TARGET Antibiotics Toolkit. Royal College of General Practitioners. 2020.https://www.rcgp.org.uk/clinical-and-research/resources/toolkits/amr/target-antibiotics-toolkit.aspx (accessed Jul 2021).
- 11Johnson AP, Lewis R, Wickens HJ. Appropriateness of antibiotic prescribing in English primary care. Journal of Antimicrobial Chemotherapy 2018;73.https://academic.oup.com/jac/article/73/suppl_2/i1/4841823
- 12Cooley N. How to screen an antibiotic prescription. Clinical Pharmacist 2010;2:217–20.https://www.pharmaceutical-journal.com/download?ac=1067532
- 13Colledge N, Walker B, Ralston S. Davidson’s principles and practice of medicine. 21st ed. Edinburgh: : Churchill and Livingstone Elsevier 2010.
- 14Marshall JC, al Naqbi A. Principles of Source Control in the Management of Sepsis. Critical Care Nursing Clinics of North America 2011;23:99–114. doi:10.1016/j.ccell.2010.12.006
- 15Antibiotics. NHS Inform. 2020.https://www.nhsinform.scot/tests-and-treatments/medicines-and-medical-aids/types-of-medicine/antibiotics (accessed Jul 2021).
- 16British National Formulary (BNF) [online]. Joint Formulary Committee. 2021.https://bnf.nice.org.uk/drug/ (accessed Jul 2021).
- 17Case-based learning: recognising sepsis. Pharmaceutical Journal Published Online First: 2019. doi:10.1211/pj.2019.20207107
- 18Tsao Y-T, Tsai Y-H, Liao W-T, et al. Differential Markers of Bacterial and Viral Infections in Children for Point-of-Care Testing. Trends in Molecular Medicine 2020;26:1118–32. doi:10.1016/j.molmed.2020.09.004
- 19Rhee C. Using Procalcitonin to Guide Antibiotic Therapy. Open Forum Infect Dis 2016;4:ofw249. doi:10.1093/ofid/ofw249
- 20Procalcitonin testing for diagnosing and monitoring sepsis. Diagnostics guidance [DG18]. National Institute for Health and Care Excellence. 2015.https://www.nice.org.uk/guidance/dg18/chapter/2-The-technologies (accessed Jul 2021).
- 21COVID-19 rapid guideline: antibiotics for pneumonia in adults in hospital [NG173]. . National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ng173 (accessed Jul 2021).
- 22Thomas J, Pociute A, Kevalas R, et al. Blood biomarkers differentiating viral versus bacterial pneumonia aetiology: a literature review. Ital J Pediatr 2020;46. doi:10.1186/s13052-020-0770-3
- 23Principles of initiating antimicrobial therapy and empiric prescribing. Clinical Pharmacist Published Online First: 2016. doi:10.1211/cp.2016.20201507
- 24Antibiotics – uses. NHS. 2019.https://www.nhs.uk/conditions/antibiotics/uses/ (accessed Jul 2021).
- 25Summary of antimicrobial prescribing guidance – managing common infections. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/antimicrobial%20guidance/summary-antimicrobial-prescribing-guidance.pdf (accessed Jul 2021).
- 26Brooks G, Carroll K, Butel J, et al. Jawetz, Melnick & Adelbergs Medical Microbiology. 26th ed. New York: : McGraw-Hill 2013.
- 27English surveillance programme for antimicrobial utilisation and resistance (ESPAUR): report 2019-2020. Public Health England. 2020.https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report (accessed Jul 2021).
- 28Blumenthal KG, Lu N, Zhang Y, et al. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ 2018;:k2400. doi:10.1136/bmj.k2400
- 29Schaefer C, Peters P, Miller R. Drugs in pregnancy and lactation. 3rd ed. London: : Elsevier 2007. https://www.sciencedirect.com/book/9780444520722/drugs-during-pregnancy-and-lactation?via=ihub= (accessed Jul 2021).
- 30Drugs to avoid in myasthenia gravis. Myaware. 2020.https://www.myaware.org/drugs-to-avoid (accessed Jul 2021).
- 31Safety of antibiotics in myasthenia gravis. Liverpool University Hospitals NHS Foundation Trust. 2019.https://secure.rlbuht.nhs.uk/sites/Antibiotic/SitePages/Antimicrobials/Myasthenia%20gravis.aspx (accessed Jul 2021).
- 32Clinical Knowledge Summaries: Acute kidney injury. National Institute for Health and Care Excellence. 2018.https://cks.nice.org.uk/topics/acute-kidney-injury/ (accessed Jul 2021).
- 33Ashley C, Currie A. The Renal Drug Handbook. 3rd ed. Oxford: : Radcliffe Publishing 2009. http://www.gicu.sgul.ac.uk/resources-for-current-staff/supplementary-inpatient-prescription-charts/renalbook.pdf
- 34Blot S, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 2014;77:3–11. doi:10.1016/j.addr.2014.07.006
- 35Daniels R, Nutbeam T. The Sepsis Manual. 5th ed. Birmingham: : United Kingdom Sepsis Trust 2019. https://sepsistrust.org/wp-content/uploads/2020/01/5th-Edition-manual-080120.pdf
- 36Phe K, Heil EL, Tam VH. Optimizing Pharmacokinetics-Pharmacodynamics of Antimicrobial Management in Patients with Sepsis: A Review. The Journal of Infectious Diseases 2020;222:S132–41. doi:10.1093/infdis/jiaa118
- 37Cobussen M, Haeseker MB, Stoffers J, et al. Renal safety of a single dose of gentamicin in patients with sepsis in the emergency department. Clinical Microbiology and Infection 2021;27:717–23. doi:10.1016/j.cmi.2020.06.030
- 38Meng L, Mui E, Holubar MK, et al. Comprehensive Guidance for Antibiotic Dosing in Obese Adults. Pharmacotherapy 2017;37:1415–31. doi:10.1002/phar.2023
- 39How should antibiotics be dosed in obesity? . UKMI/ Association of Scottish Antimicrobial Pharmacists and Healthcare Improvement Scotland. 2017.https://www.gloshospitals.nhs.uk/media/documents/Obese_Dosing_2016_update.pdf (accessed Jul 2021).
- 40Maricelle O. Duration of Antibiotic Therapy: General Principles. Health System Edition 2017;6.https://www.pharmacytimes.com/view/duration-of-antibiotic-therapy-general-principles
- 41Cyriac J, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother 2014;5:83. doi:10.4103/0976-500x.130042
- 42Olusoga A, Howard P, Atack K, et al. Antimicrobial stewardship in a large teaching hospital: is the reassessment of intravenous antibiotics on our safety radar? The journey so far. Amsterdam, Netherlands: 2019.
- 43Intravenous to Oral Antimicrobial Therapy Review and Switch (IVOS). Leeds Health Pathways. http://nww.lhp.leedsth.nhs.uk/antimicrobials/IVOS.pdf (accessed Jul 2021).
- 44Shorter = Better: Stewardship . Spellberg B. 2020.https://www.bradspellberg.com/shorter-is-better (accessed Jul 2021).
- 45Ashiru-Oredope D. How pharmacists can help in tackling antimicrobial resistance. Public health matters. 2016.https://publichealthmatters.blog.gov.uk/2016/11/09/how-pharmacists-can-help-in-tackling-antimicrobial-resistance/ (accessed Jul 2021).