Shutterstock.com
Any pharmacy that provides drug treatment services can now supply nasal naloxone to individuals without the need for a prescription, patient group direction or patient specific direction, following an amendment to the Human Medicines Regulations 2015.
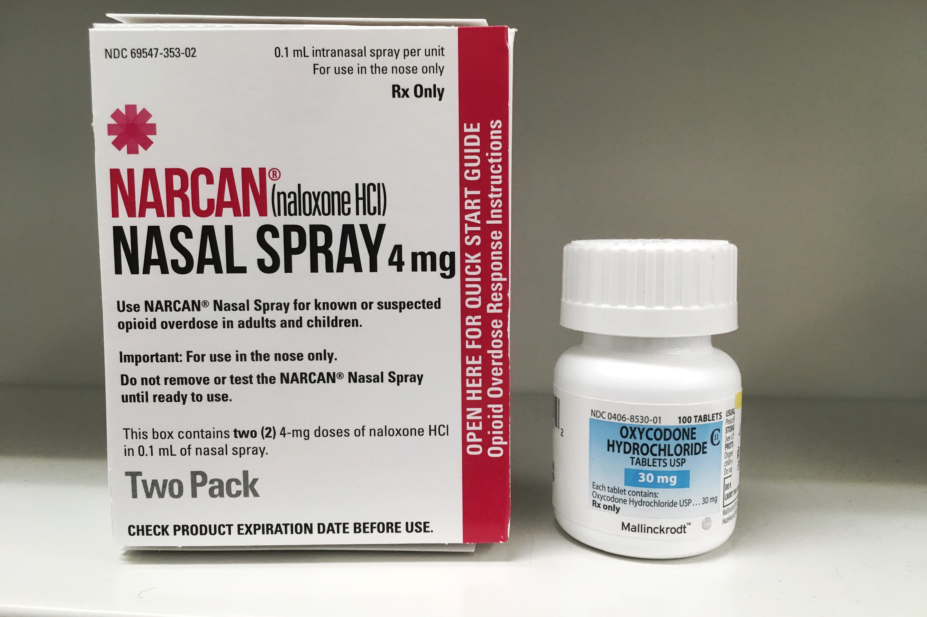
Naloxone is the emergency antidote for overdoses caused by heroin and other opioids, such as methadone, morphine and fentanyl.
Under regulations that came into force in October 2015, people working in drug treatment services could, as part of their role, supply injectable naloxone to anyone for the purpose of saving a life in an emergency. This meant that drug services could supply naloxone to an outreach worker, hostel manager, carer, friend or family member, or a drug user at risk in the event of an emergency.
These regulations were amended on 18 February 2019 to include nasal naloxone.
The types of drug treatment services that can supply naloxone are those provided by, on behalf of, or under arrangements made by an NHS body, local authority, a Public Health Agency, or Public Health England.
This includes pharmacy needle exchange programmes if they are commissioned by local authorities or the NHS, and pharmacies providing drug treatment such as opioid substitution treatments through supervised consumption prison drug services.
Current clinical guidance recommends that drug services provide suitable training and advice to people when supplying naloxone.

