Garo / Phanie / Science Photo Library
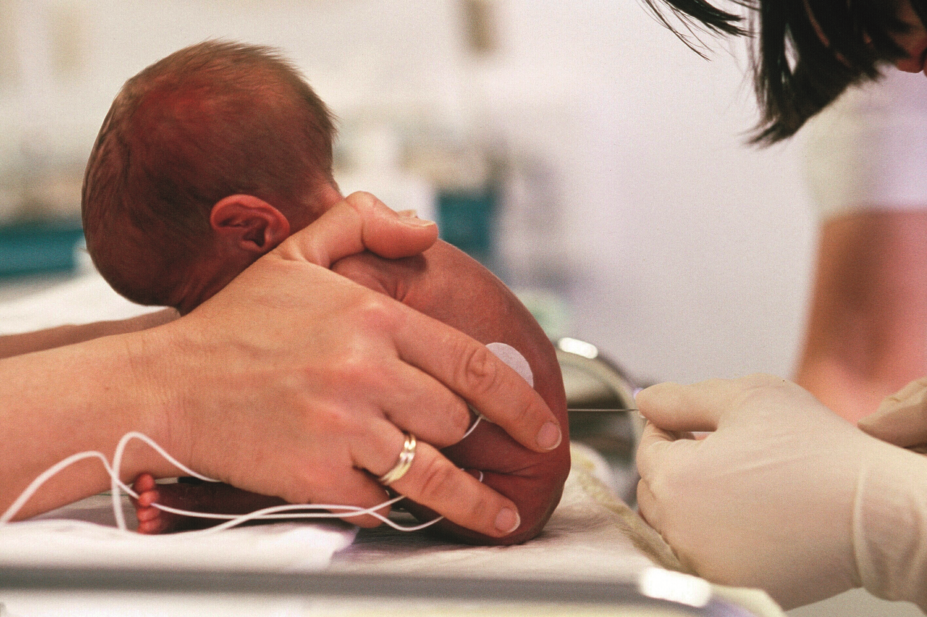
Infantile-onset spinal muscular atrophy (SMA) is the most severe form of the disease, and most babies diagnosed with the condition don’t live beyond two years without breathing and feeding support.
Nusinersen is an antisense drug that redirects a ‘back-up’ gene (SMN2) to produce a protein that is deficient in babies with SMA.
In a phase II open-label trial, researchers explored the effect of nusinersen delivered by lumbar puncture to 20 babies with SMA aged between three weeks and seven months.
An interim analysis, published in The Lancet
[
1]
(online, 6 December 2016), shows the drug was well tolerated and there were no safety concerns over a maximum follow-up of 32 weeks.
The authors also observed improvement in muscle skills and function in the majority of babies and 16 babies survived without needing permanent breathing support.
The researchers conclude that their findings provide proof of principle for the use of antisense drugs such as nusinersen in neurological disorders.
References
[1] Finkel RS, Chiriboga CA, Vajsar J et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase II, open-label, dose-escalation study. The Lancet 2016. doi: 10.1016/S0140-6736(16)31408-8