Shutterstock.com
Barcode errors on medicines “pose critical patient safety risks” and could have “potentially fatal consequences,” Henrietta Hughes has warned.
The patient safety commissioner for England’s warning comes as a petition launched by pharmacists report growing problems with barcode data errors and missing 2D barcodes on UK medicine packs.
The issue has been highlighted by several ‘Class 4 medicines defect notifications’ during 2025 that were linked to barcode or labelling problems, including fexofenadine hydrochloride tablets in August 2025, and simvastatin tablets in July 2025.
A Class 4 Medicines Defect Notification is a ‘caution in use’ notice issued by the Medicines and Healthcare Regulatory Agency (MHRA) for defects such as errors in packaging or printed materials.
In comments made to The Pharmaceutical Journal, Hughes said: “Mislabelled packs can result in wrong medicines, dosages or formulations reaching patients with potentially harmful or even fatal consequences.
“Barcode accuracy, in modern healthcare, is literally life or death.”
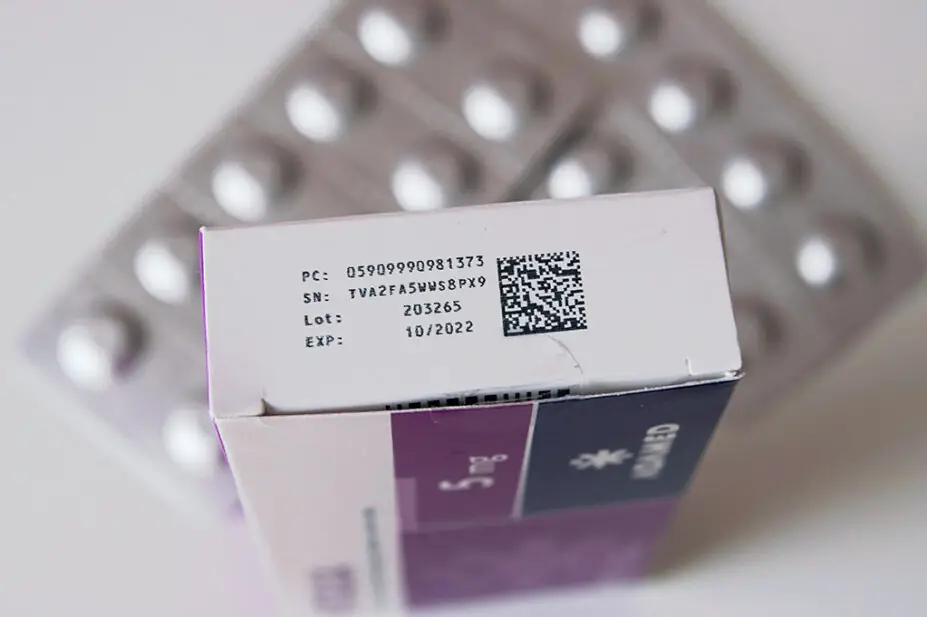
Under the EU’s Falsified Medicines Directive (FMD), which was in place since 2013, 2D barcodes following the GS1 standards and containing the Global Trade Item Number (GTIN), medicine packs in the UK were required to include a batch number, expiry date and unique serial number.
However, since the FMD no longer applied in Great Britain from 31 December 2020, when the UK exited the EU — and in Northern Ireland following the Windsor Framework changes introduced from 1 January 2025 — manufacturers are no longer required to include 2D barcodes on UK-only packs, meaning expiry date and batch number information is lost.
In June 2025, the UK’s four chief pharmaceutical officers wrote an open letter urging pharmaceutical companies in the UK to continue 2D barcodes on all medicine packs “given the safety and operational benefits”, despite this no longer being mandatory.
And, in October 2025, 32 pharmacy professionals across NHS trusts and digital groups wrote to Lawrence Tallon, chief executive of the MHRA, and Alison Cave, its chief safety officer, urging the Agency to mandate 2D barcodes and include GTINs in the medicine licensing process, especially given the government’s shift from ‘analogue to digital’ in the NHS ten-year plan.
Iain Davidson, chief pharmacist at the Royal Cornwall Hospitals NHS Trust and one of the signatories to the letter, seen by The Pharmaceutical Journal: “If you remove traceability across the supply chain, you can’t do recalls as effectively. The whole point of barcode medication administration is that it’s the right product for the right patient at the right dose.”
Another signatory, Richard Bowers, lead pharmacist of innovation at Leeds Teaching Hospitals NHS Trust (LTHT), warned barcode inconsistency was undermining efforts to digitise medicine supply and administration.
“Our vision at LTHT is to implement automated and integrated systems for the management and supply of medicines to our patients, and this hinges on medicine packs having GS1 barcodes,” he said.
“Manually bypassing this process for a significant proportion of medicines due to lack of barcodes negates the safety benefits and calls into question the robustness of the whole process.”
Kristy McGlynn, a digital medicines pharmacist at Manchester University NHS Foundation Trust, commented: “Medications are coming through the supply chain with barcodes that are not listed on the Dictionary of Medicines and Devices, therefore are not uploaded into the integrated pharmacy stock control and EPMA/EPR database.
“This presents a challenge for drug recalls if the lot and expiry aren’t captured at goods receipt and during barcode medication administration.”
“Additionally, we have seen increases incidences of barcode recycling or incorrect barcodes printed on the packaging in which we have had to report to the MHRA, resulting in Caution In Use notices,” she added.
A spokesperson for the MHRA told The Pharmaceutical Journal it was “aware of the current reported issues in relation to barcode errors” but noted that it does not regulate GTINs, adding “the management of these is not within our remit”.
The regulator said it was “considering broader industry feedback” and was “engaged with healthcare professionals and stakeholders who use automated systems to better understand the impact of the incorrect data”.
It added that it would “continue to monitor the situation”.
A spokesperson for Medicines UK, which represents generic and biosimilars manufacturers, said that some manufacturers dispensed with “the serialisation aspect of FMD” post-Brexit to speed production, and argued that counterfeiting was rare for low-cost generic medicines.
“Dispensing errors can occur for a number of different reasons, which are complex and interrelated,” they said.
“Manufacturers play their part to help avoid dispensing errors by carefully designing and user testing their packaging designs, which have to be approved by the MHRA.”
David Watson, executive director of patient access at the Association of the British Pharmaceutical Industry (ABPI), told The Pharmaceutical Journal that there was “an opportunity for further discussion” to ensure a system that “protects patient safety and supporting supply chain resilience”.
The Department of Health and Social Care (DHSC) was contacted but declined to comment for this article.


