Shutterstock.com
For some, it begins with fatigue and nausea. For others, a gnawing pain in their bones. These and other disparate symptoms can herald the same culprit: multiple myeloma, an incurable cancer of plasma cells.
Cancer learning ‘hub’
Pharmacists are playing an increasingly important role in supporting patients with cancer, working within multidisciplinary teams and improving outcomes.
However, in a rapidly evolving field with numbers of new cancer medicines is increasing and the potential for adverse effects, it is now more important than ever for pharmacists to have a solid understanding of the principles of cancer biology, its diagnosis and approaches to treatment and prevention.
This new collection of cancer content, brought to you in partnership with BeOne Medicines, provides access to educational resources that support professional development for improved patient
Plasma cells are a type of white blood cell residing in the bone marrow, where they normally churn out antibodies to safeguard the body against infection. However, in multiple myeloma, malignant plasma cells turn out useless antibodies, known as paraprotein.
The build-up of malignant plasma cells in the bone marrow, and presence of paraprotein, causes problems in the regions of the body where bone marrow is normally active, such as the bones of the spine, pelvis and ribcage. Multiple myeloma can also cause damage to the kidneys and interfere with the immune system, leading to recurrent infection.
In the UK, about 5,700 people are diagnosed with multiple myeloma each year, and there are nearly 18,000 living with the disease. Thankfully, an arsenal of treatments stands at the ready. Chemotherapy agents are used with the aim of killing the malignant plasma cells and, for some patients, this may be followed by a stem cell transplant to give a fresh start to bone marrow.
But multiple myeloma is hard to beat definitively, which means patients in remission will eventually relapse when malignant cells resurface. As one treatment fails, another is tried and, the further into the roster of treatment a patient goes, the poorer the prognosis.
The natural history of myeloma is such that all patients will relapse
Noopur Raje
“More than 90–95% of people will actually respond to initial therapy, but the natural history of myeloma is such that all patients will relapse,” explains Noopur Raje, clinical director of the Center for Multiple Myeloma at Massachusetts General Hospital in Boston, United States.
“There’s attrition as they go through their disease course and disease progression.
“Patients will die of myeloma or they could die of infections, kidney-related problems, bone-related problems. There’s a lot of morbidity and mortality associated with some of the end organ damage from multiple myeloma,” she adds.
For those who have come to the end of their treatment options, a novel approach beckons: chimeric antigen receptor T (CAR-T) cells. CAR-T therapy involves programming a patient’s own T cells – a type of white blood cell that has a critical role in orchestrating the immune response – to recognise malignant cells as pathogens, thus harnessing the force of the immune system to target and kill them.
The responses we’re seeing with CAR-T in multiple myeloma are way beyond our wildest dreams
Jesus Berdeja
“The responses we’re seeing with CAR-T in multiple myeloma are way beyond our wildest dreams,” says Jesus Berdeja , director of myeloma research at the Sarah Cannon Cancer Institute in Nashville, Tennessee, who is involved in these clinical trials. “I think CAR-T is here to stay, and we need to figure out how to maximise its effect and minimise downsides and toxicity.”
CAR-T so far
CAR-T therapies have already racked up successes in other blood cancers. Since 2018, two CAR-T products: tisagenlecleucel (Kymriah; Novartis) and axicabtagene ciloleucel (Yescarta; Gilead), have been licensed in the UK, through the Cancer Drugs Fund, for treating relapsed or refractory types of B cell lymphomas. Approval for use of these products in follicular lymphoma is expected in 2021. In December 2020, another therapy, brexucabtagene autoleucl (Tecartus; Gilead), was awarded a conditional marketing authorisation for use in mantle cell lymphoma (a rare type of B cell non-Hodgkin lymphoma) by the European Medicines Agency (EMA). And, yet another, lisocabtagene maraleucel (Bristol Myers Squibb), showed benefits in a clinical trial of B cell lymphoma published in September 2020[1].
The results for multiple myeloma not only add to this good news but signal an important expansion of CAR-T into the treatment of other types of cancer.
So far, all licensed CAR-T products target CD19, a molecule highly expressed on the malignant cells in B cell lymphomas. But in multiple myeloma trials, most CAR-T products target B-cell maturation antigen (BCMA), a cell surface protein expressed on mature B cells and plasma cells, with higher levels on malignant cells.
Like all CAR-T therapies, the BCMA-targeting CAR-T cells have their risks, since they involve tinkering with the immune system and can also target healthy tissue. But the response rates have surpassed expectations, and one product, idecabtagene vicleucel (ide-cel, bb2121; Bristol Myers Squibb and bluebird bio), is now under review for regulatory approval in the United States.
This success with a new type of CAR-T therapy suggests that, with careful design and the right targets, CAR-T has the potential to treat even more cancers, including solid tumours (see Box). And, as these different applications accumulate and demand for CAR-T grows, pharmacists are readying themselves by standardising protocols for handling the cell products and developing guidance around CAR-T therapies.
“It’s really important that the whole pharmacy workforce is aware of CAR-T and the impact of this treatment, and the potential side effects and interactions to look out for,” says Alice Mason, lead pharmacist in Advanced Therapy Medicinal Products (ATMP) at the Royal Marsden NHS Foundation Trust in London.
It’s really important that the whole pharmacy workforce is aware of CAR-T and the impact of this treatment
Alice Mason
“If a patient who recently had CAR-T turns up with a headache, then even the community pharmacist will need to have an awareness of these patients,” she adds.
Designing a living drug
Unlike chemotherapy treatments, CAR-T offers a personalised, albeit involved, approach.
T cells are collected from a patient’s blood and genetically engineered to produce CARs on their surface. These receptors enable the T cells to recognise and attach to specific proteins, or antigens, on tumour cells.
After engineering, the T cells are then expanded in culture before being infused back into the patient.
If all goes to plan, when the CAR-T cell encounters the target molecule on a malignant cell in the patient’s body, it is able to bind to it and kill it.
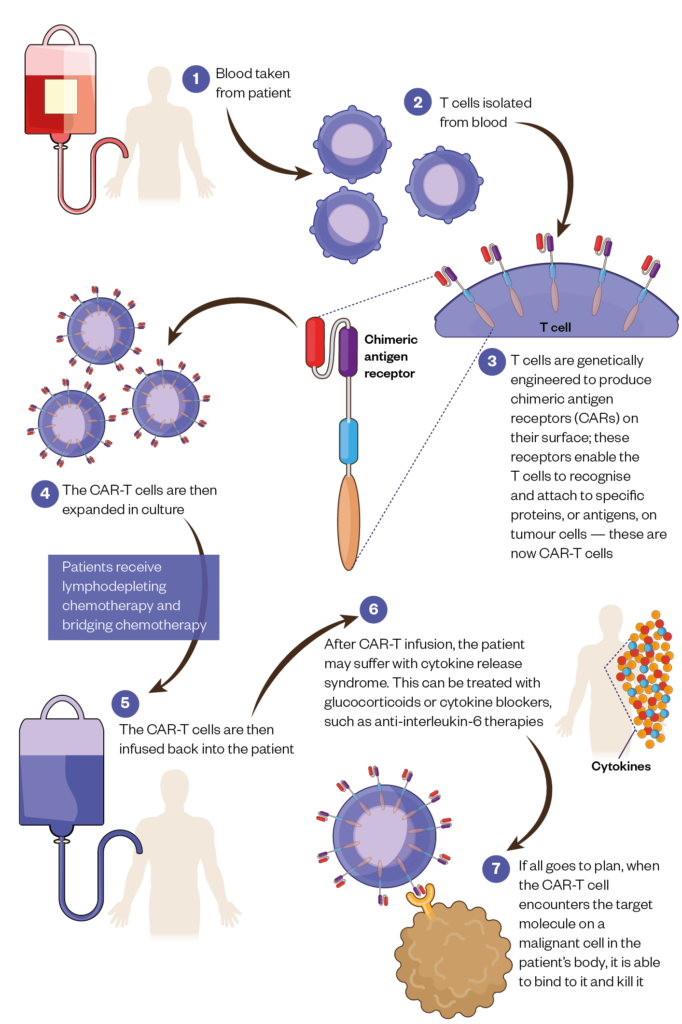
The procedure is not without its risks and recipients require careful monitoring.
Prior to receiving CAR-T therapy, patients receive lymphodepleting chemotherapy, which helps to ensure there is a favourable immune environment for the CAR-T cells to replicate further once they are infused. While waiting for their CAR-T cells to be manufactured, patients will often also need bridging chemotherapy to help keep their disease in check.
Once they receive their infusion of CAR-T cells, patients may suffer cytokine release syndrome, a body-wide inflammatory response that can be lethal if not caught in time. This may be treated by tamping down the immune system’s over-reaction with glucocorticoids or cytokine blockers, such as anti-interleukin-6 therapies[2].
Signs of neurotoxicity, such as confusion or lethargy, can also emerge in patients, although it remains unclear if these symptoms are the result of cytokine release syndrome or from CAR-T cells somehow crossing the blood-brain barrier. And, because CAR-T’s targets are not necessarily found solely on malignant cells, there can also be “on-target, off-tumour” toxicities that kill off healthy cells. To limit toxicity, alternative CAR-T designs are being looked at[3].
One example is an emergency ‘safety switch’ receptor that is co-expressed with the CAR on a T cell. If a patient runs into trouble, a drug directed at the receptor can be given to selectively ablate all CAR-T cells; for example, cetuximab will bind to a truncated version of the epidermal growth factor receptor, which then triggers the death of CAR-T cells carrying it[4].
Researchers are also aiming to shorten the time it takes to prepare CAR-T therapy by developing allogeneic T cells. These are T cells collected from healthy people, transfected with a CAR, and stored away until needed.
Such ‘off-the-shelf’ cells require some matching between donor and patient, and are currently in clinical trials. But, if proven, they could substantially shorten the waiting time for patients who are very unwell, and potentially stave off T cell exhaustion – a reduced ability to kill cells, recruit other T cells, or proliferate – that sets in for ‘autologous’ CAR-T made from the T cells of patients.
CAR-T’s new in-road
Fifteen clinical trials are now investigating CAR-T therapies that target BCMA for multiple myeloma[5].
The product furthest along is idecabtagene vicleucel, which Raje is investigating: a phase I trial published in 2019 found that it commonly induced adverse — though treatable — effects and toxicities among 33 patients with relapsed or refractory disease[6].
For example, 76% of participants experienced cytokine release syndrome, and 42% showed signs of neurologic toxicity, such as confusion or lethargy.
Yet, the risks appear to be worth it. In 85% of patients, idecabtagene vicleucel was found to induce a clinical response – including a reduction in abnormal antibody production – in malignant cells in the bone marrow and in the size of skeletal lesions. Updated findings with more patients concluded with a similar level of efficacy. The product is now under review by the US Food and Drug Administration and the EMA.
Another product, ciltacabtagene autoleucel (cilta-cel; Janssen), is following closely behind. Previous trials have found it to be safe in China, and it has qualified for an expedited review by several regulatory agencies, including the EMA[7,8].
A trial of the same CAR is now under way in the United States, with phase Ib/II results presented at a meeting in December 2020 showing a favorable safety profile and a nearly 95% response rate[9].
“CAR-T annihilates basically every BCMA-expressing cell within days in the patients that do respond,” says Berdeja, who is involved in the trial. “This is something we’ve never seen before.”
CAR-T annihilates basically every BCMA-expressing cell within days in the patients that do respond
Jesus Berdeja
However, some patients with multiple myeloma do still relapse after CAR-T therapy.
While the reasons for this are unclear, other trials are testing CAR-T directed at other antigens, sometimes coupled with the anti-BCMA CAR, as malignant cells can evolve away from expressing a certain target molecule. But trials in the very sick relapsed or refractory patient population may underestimate CAR-T’s potential.
Prior chemotherapy treatments take their toll on T cells, so that by the time CAR-T therapy is tried, T cells are less able to mount the required responses.
Giving idecabtagene vicleucel earlier in the course of the disease is now an area of study. But, even if earlier treatment works well, deciding on the best course of treatment will involve weighing up the risks and benefits.
Standard therapies can give patients 10–15 years of survival, without the risk of dying from severe cytokine release syndrome associated with CAR-T treatment.
Therefore, high-risk multiple myeloma patients who do not respond well to standard treatments may be the best candidates for early CAR-T treatment, Berdeja says. For them, even if CAR-T therapies are no better than a standard maintenance medicine, they would not need to be taken continuously.
“Ultimately, it would be nice if you had a therapy that’s one and done,” he adds.
If CAR-T therapy could avert downstream treatments for enough patients, it’s high list price – around £28,000 per patient, although the NHS has negotiated significant discounts for both Yescarta and Kymriah – could actually be cost-effective. Analyses of CAR-T therapies for lymphomas suggest this is not yet the case, and the National Institute for Health and Care Excellence still needs to carry out further data collection to address uncertainties in the clinical and cost-effectiveness evidence. However, the potential for the therapies to save the lives of many patients who have run out of options must be weighed as well[10].
Box: Solid vistas
Researchers are also exploring CAR-T therapies for solid tumours, although these products are not as advanced in their development as those for blood cancers[11].
Solid tumour cancers are hugely diverse, which means each type will have its own set of candidate molecules for targeting. Solid tumours also pose an especially challenging situation for CAR-T cells, because it is harder for the T-cells to access malignant cells within a tumour, and the tumour itself creates an unfriendly microenvironment that weakens T cells.
“So, the T cells have a lot harder job to get to the tumour cells, and then to persist long enough to continue to have activity,” explains Julie Park, a professor of paediatrics at Seattle Children’s Hospital in Seattle, Washington. There are many strategies for overcoming these challenges, and some positive signs have emerged, most recently in a phase I trial published in November 2020[12].
This study reported that CAR-T therapy directed at GD2, a protein expressed on the surface of some tumors of the nervous system, was well tolerated and did not have significant on-target off-tumour toxicity in paediatric neuroblastoma patients.
Although none of the patients met the criteria for a bonafide clinical response, the researchers did find signs of tumour regression.
Finding a way to get T cells to persist may be one way to enhance the effects of CAR-T in solid tumours, says Park. Park and colleagues developed the first solid tumour CAR-T trial for children with neuroblastoma, targeting L1-CAM, a glycoprotein found on the surface of neurons, and she is currently involved in CAR-T trials with different target antigens to treat recurrent or refractory solid tumours in children[13].
To enhance T cell persistence, the CAR-T cells in these trials have an additional receptor that binds a ligand present on B cells, in the hope that this will stimulate CAR-T cell proliferation.
Looking to the future
So far, nine hospitals in the UK are accredited to prepare and deliver CAR-T therapy, but this network is expected to grow as the applications for CAR-T expand. The Pan UK Pharmacy Working Group for ATMPs had pharmacists from existing CAR-T centres produce institutional readiness documents that can be shared as CAR-T centres multiply. These include checklists for handling CAR-T cells and medicines restrictions for the patients receiving them[14,15].
‘We’re working collaboratively to learn from the experience of each current CAR-T centre to try to have a more standardised approach across the UK as opposed to every trust doing their own thing,” Mason says.
In the meantime, patients living far away from these hospitals will have to continue to make the journey back and forth, and require coordinated care between CAR-T centres and local clinics. This means all pharmacists, not just those in the hospitals delivering CAR-T, need to familiarise themselves with CAR-T therapies, Mason adds.
“This is a very new area for pharmacy and we’re working together to help guide the CAR-T landscape in the UK,” she says.
- This article was corrected on 5 March 2021 to refer to anti-interleukin-6 therapies, not interleukin 6.
- 1Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet 2020;:839–52. doi:10.1016/s0140-6736(20)31366-0
- 2Fajgenbaum D, June C. Cytokine Storm. N Engl J Med 2020;383:2255–73. doi:10.1056/NEJMra2026131
- 3Cortés-Hernández A, Alvarez-Salazar EK, Soldevila G. Chimeric Antigen Receptor (CAR) T Cell Therapy for Cancer. Challenges and Opportunities: An Overview. In: Methods in Molecular Biology. Springer US 2020. 219–44. doi:10.1007/978-1-0716-0759-6_14
- 4Paszkiewicz PJ, Fräßle SP, Srivastava S, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. Journal of Clinical Investigation 2016;126:4262–72. doi:10.1172/jci84813
- 5Shah N, Chari A, Scott E, et al. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia 2020;34:985–1005. doi:10.1038/s41375-020-0734-z
- 6Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med 2019;380:1726–37. doi:10.1056/nejmoa1817226
- 7Zhao W-H, Liu J, Wang B-Y, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol 2018;11. doi:10.1186/s13045-018-0681-6
- 8Xu J, Chen L-J, Yang S-S, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci USA 2019;116:9543–51. doi:10.1073/pnas.1819745116
- 9Madduri D, Berdeja J, Usmani S. CARTITUDE-1: Phase 1b/2 Study of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen–Directed Chimeric Antigen Receptor T Cell Therapy, in Relapsed/Refractory Multiple Myeloma. . American Society of Haematology . 2020.https://ash.confex.com/ash/2020/webprogram/Paper136307.html (accessed 17 Feb 2021).
- 10Lin JK, Muffly LS, Spinner MA, et al. Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Multiply Relapsed or Refractory Adult Large B-Cell Lymphoma. JCO 2019;37:2105–19. doi:10.1200/jco.18.02079
- 11Wagner J, Wickman E, DeRenzo C, et al. CAR T Cell Therapy for Solid Tumors: Bright Future or Dark Reality? Molecular Therapy 2020;28:2320–39. doi:10.1016/j.ymthe.2020.09.015
- 12Straathof K, Flutter B, Wallace R, et al. Antitumor activity without on-target off-tumor toxicity of GD2–chimeric antigen receptor T cells in patients with neuroblastoma. Sci Transl Med 2020;12:eabd6169. doi:10.1126/scitranslmed.abd6169
- 13Park JR, DiGiusto DL, Slovak M, et al. Adoptive Transfer of Chimeric Antigen Receptor Re-directed Cytolytic T Lymphocyte Clones in Patients with Neuroblastoma. Molecular Therapy 2007;15:825–33. doi:10.1038/sj.mt.6300104
- 14Pharmacy Institutional Readiness for Marketed CAR-T Therapy: Checklists for Pharmacy Services. . Specialist Pharmacy Service and Northern Alliance. 2020.https://www.sps.nhs.uk/wp-content/uploads/2018/10/Pharmacy-Institutional-Readiness-for-Marketed-CAR-T-Therapy-Guidance-for-Chief-Pharmacists-V4.pdf (accessed 17 Feb 2021).
- 15Medication Restrictions for Patients Having CAR-T Cell Therapy. . Pan UK Pharmacy Working Group for ATMPs. 2020.https://www.sps.nhs.uk/wp-content/uploads/2020/01/Medication-Restrictions-for-Patients-having-CAR-T-Therapy-Aug-2020.pdf (accessed 17 Feb 2021).


