JL / The Pharmaceutical Journal
The leopard tortoise, found throughout Africa, has a shell with steep, almost vertical, sides and a low centre of gravity that allows it to right itself if it rolls onto its back. It is the shape of this tortoise shell that inspired researchers to develop a capsule device that can reorient itself in the dynamic environment of the stomach, and allow a small needle made of insulin to be injected directly into the stomach wall.
This capsule, developed by researchers at Massachusetts Institute of Technology (MIT), is just one of several promising strategies for delivering insulin orally — a goal the pharmaceutical industry has been striving to achieve since early unsuccessful attempts in the 1920s.
Insulin is currently injected by nearly 200 million people with diabetes worldwide[1]
. Since its discovery almost a century ago, there have been several advances in its use, from the introduction of human insulin to the production of insulin pens and pumps, and analogue insulins — all of which have had a huge impact on the lives of people with diabetes. However, the development of a commercially available insulin pill has remained elusive.
Obstacles to oral delivery
The gastrointestinal tract is designed not to absorb large molecules and so these drug molecules are essentially facing a wall
Oral delivery of insulin and other biologics is hampered by their large size compared with small-molecule drugs. The oral route is not designed to let large macromolecules into the blood stream; instead, the gastrointestinal (GI) tract is engineered to do the exact opposite (see Figure 1a).
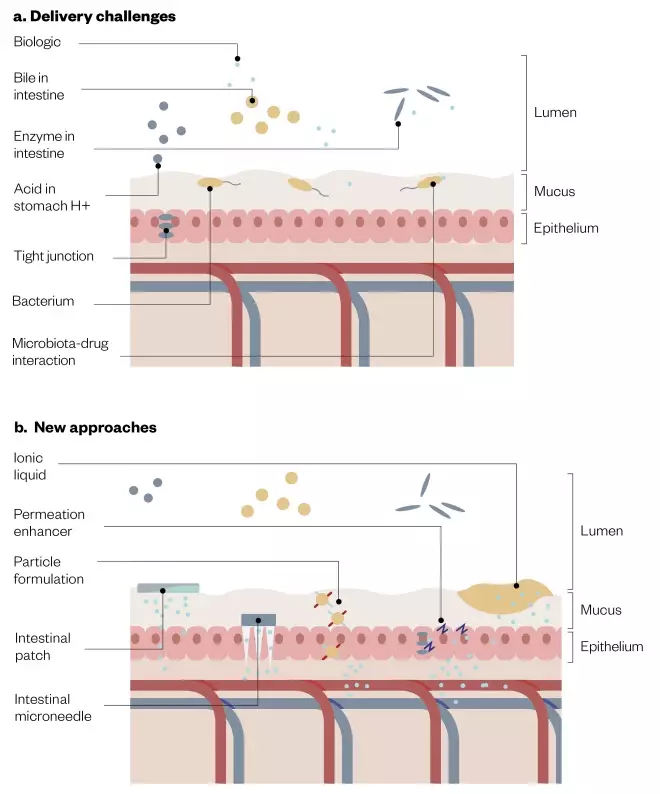
Figure 1: Barriers to delivery and new oral delivery sytems
Source: JL / The Pharmaceutical Journal. Adapted with permission from Springer Nature. Anselmo AC, Gokarn Y, Mitragotri S. Nat Rev Drug Discov 2018;18(1):19–40[7]
New approaches to overcome barriers to oral delivery of biologics, such as insulin, include permeation enhancers, intestinal patches and microneedles.
“[It] is designed not to absorb large molecules and so these [drug] molecules are essentially facing a wall,” explains Giovanni Traverso, a doctor and researcher at Harvard Medical School and visiting scientist at MIT, who is part of the team developing the self-orienting capsule.
In order to be absorbed by the body, a drug molecule must first pass through the gut’s mucus barrier — composed of densely glycosylated proteins — and then through the gut epithelium. The single layer of epithelial cells lining both the small intestine and the large intestine acts as a physical barrier, with tight junctions between adjacent cells limiting the passage of molecules and ions. Most materials must enter the cells by diffusion or active transport to pass through the tissue.
“Anything larger than a dipeptide or a small molecule — somewhere around 400–500Da — and it tends to get harder to deliver,” says Paul Shields, chief operating officer of New Jersey-based biopharmaceutical company Enteris BioPharma. Although Enteris is not working on insulin, it has developed several oral delivery technologies for other biologics which are currently in late-stage clinical trials. Delivering these drugs shares some of the problems encountered in delivering insulin. “Certainly by the time you get to 100kDa, you are not going to be able to deliver it orally,” he adds.
In addition, there are chemical hazards that break down biologics. “Specifically, the stomach is a very acidic environment, so the acid itself can attack the therapeutic,” explains Traverso. “There are also enzymes that break up those molecules — both proteases and nucleases.”
The population is used to taking pills — it is non-invasive and painless, so it just makes it easier for the patient
Human insulin comprises 51 amino acids and has a molecular mass of 5,808Da; however, the challenge is not just one of size, but also the variability in insulin’s bioavailability when given orally. Shields describes it as “a ridiculously bad candidate for oral delivery”.
With all of these difficulties, it is perhaps surprising that there has been a push towards finding ways to administer insulin and other biologics orally. “The population is used to taking pills — it is non-invasive and painless, so it just makes it easier for the patient,” says Samir Mitragotri, a bioengineer and drug delivery expert at Harvard University, Massachusetts. And, according to Traverso, in addition to patient preference, the absence of oral medicines does have clinical consequences. “It has been estimated that starting insulin is delayed by about eight years [for people with diabetes].” This may reflect the reluctance of professionals to intensify treatment.
In 2016, the Danish pharmaceutical giant Novo Nordisk announced that it had shelved plans to bring the world’s first oral insulin to market. Developed in collaboration with Robert Langer, who heads a biomedical research laboratory at MIT, the company has since shown that although its oral formulation was effective, it did not prove to be economical. Because much less of the insulin is absorbed, the oral formulation required much larger quantities of the drug.
Nevertheless, Novo Nordisk has not given up on developing an oral biologic for diabetes and is working on an oral glucagon-like peptide 1 (GLP-1) receptor agonist — a relatively new group of injectable drugs for type 2 diabetes mellitus. Injectable semaglutide (Ozempic, Novo Nordisk) mimics the function of the GLP-1 hormone through lowering post-meal blood glucose levels. The company’s new daily semaglutide tablet has been through multiple positive trials — it was filed for approval with the US Food and Drug Administration in March 2019 and the European Medicines Agency the following month. The Israeli biotechnology company Oramed Pharmaceuticals is also developing an oral GLP-1 analogue capsule known as ORMD-0901.
Others are continuing the quest for oral insulin (see Figure 1b), which may have several advantages over the injectable version. For example, intestinally absorbed insulin better mimics insulin’s natural location and gradients in the body by passing through the liver before entering the bloodstream. Oramed has completed multiple phase II trials with its oral human insulin capsule (ORMD-0801) and several other oral insulins are under development (see Table).
| Name (company) | Delivery approach | Biologic application | Indication | Clinical trials |
|---|---|---|---|---|
| Source: Nat Rev Drug Discov [7] | ||||
| Capsulin OAD (Diabetology) | Capsule using the proprietary technology platform Axcess | Insulin | Type 2 diabetes mellitus (T2DM) | Phase II |
| NN9924 (Novo Nordisk) | Tablet with absorption-enhancing excipients | Semaglutide | T2DM | >25 trials, including phase III |
| ORMD-0801 (Oramed) | Oral insulin capsule that prevents enzyme degradation and enhances intestinal absorption | Insulin | Type 1 diabetes mellitus (T1DM) and T2DM | Multiple phase II |
| TTP273 (vTv Therapeutics) | Tablet | Glucagon-like peptide 1 | T2DM | Phase II |
| Tregopil; formerly IN-105 (Biocon) | Tablet | Novel oral insulin molecule | T1DM | Phase I |
| Oral HDV Insulin (Diasome) | Capsule containing insulin targeted to the liver | Insulin | T2DM | Phase II/III |
| Oshadi Icp (Oshadi Drug Administration) | Oral formulation | Insulin | T1DM | Phase II |
Enzyme inhibitors and absorption enhancers
These new oral delivery systems generally start with some form of enteric capsule that protects the drug from a low-pH environment and allows it to be released in the correct place — usually in the upper tract of the intestines. But this is not enough, explains Shields. “Even if you get it there intact, it’s not going to absorb, plus the intestines have a lot of proteases, so if you are working with a natural amino acid peptide, they will tend to chew it up.”
To overcome these barriers, new oral formulations include both enzymatic inhibitors and what are known as ‘permeation enhancers’, mixed with the biologic. Permeation enhancers include chelating agents, lipids, organic solvents and polymers, and are able to disrupt the epithelial tight junctions to allow larger molecules to pass through.
Shields is working on Enteris’s ‘Peptelligence’ formulation technology for oral delivery of biologics, which includes citric acid and a permeation enhancer based on acetyl L-carnitine, a naturally occurring metabolic transporter molecule. “The combination of the acid and the enhancer seems to moderately loosen the tight junctions of the endothelial cells temporarily,” he explains.
Oramed’s ‘POD’ (protein oral delivery) technology uses a similar approach for its oral insulin. “The main essence of our formulation is the protection of the peptide molecule from those proteases using protease inhibitors,” says Miriam Kidron, the company’s chief scientific officer. Specialised protease inhibitors seem to be preferentially attacked by proteases in the small intestine, sparing the drug molecule. An absorption enhancer supplement also facilitates the drug passing across the intestinal barrier.
Another method used to aid absorption is muco-adhesive patches. “It works like a skin patch,” says Mitragotri, who has tested the approach. “Imagine a capsule and within [it] there are about half a dozen or so patches, and each patch has the drug dissolved in a [polymer] matrix.” When the capsule opens, the patches stick to the surface of the intestine and create protected, high-concentration drug depots, which drive drug diffusion across the epithelium into the blood circulation. Mitragotri’s group has used patches to deliver therapeutic doses of insulin in rats, with a corresponding and dose-dependent drop in blood glucose[2]
.
However, the size of the molecule is always going to limit these types of oral delivery. Driton Vllasaliu, a pharmaceutical scientist at King’s College London who is developing a different approach, suggests there could be safety issues with some of the strategies that use enzyme inhibitors and absorption enhancers. “There are concerns related to the safety of the materials used and the non-specific increase in intestinal permeability, which could potentially result in unwanted materials present in the gut lumen entering the systemic circulation,” he says.
Transcellular carriers
Instead of increasing intestinal permeability, Vllasaliu and others are making use of receptor-mediated transport across the epithelium using various transcellular carriers, including nanoparticles.
“Researchers have been designing carriers that are capable of ‘hijacking’ biological transport processes to shuttle the biological drug across the mucosa,” explains Vllasaliu. The process, known as transcytosis, allows various macromolecules to be drawn across the cell membrane and ejected on the other side via a vesicle.
In animal tests, orally administered nanoparticles containing insulin showed more than a ten-fold increase in absorption compared with non-targeted nanoparticles
“We are identifying transport-enabling peptides which, when attached to biologics-carrying nanocarriers, improve their transport across the intestinal mucosa,” he explains. “These peptides interact with biological receptors expressed in the gut epithelium that participate in physiological transport of material across the intestinal mucosa.”
This strategy has also been used by a team from MIT and Harvard to target the neonatal Fc receptor (FcRn), a protein found on cell surfaces to mediate the transport of antibodies. The team used biodegradable and biocompatible polymer nanoparticles (poly(lactic acid)–b-poly(ethylene glycol)) conjugated with the corresponding FcRn-targeted antibody portion. In animal tests, orally administered nanoparticles containing insulin showed more than a ten-fold increase in absorption compared with non-targeted nanoparticles, and enough to administer a clinically relevant insulin dose[3]
.
Novo Nordisk’s oral semaglutide formulation also makes use of transcellular carriers, via a technology it has licensed from drug delivery firm Emisphere, based in New Jersey. Emisphere has developed a library of approximately 4,000 potential carrier molecules.
Delivery devices
This is a painless process due to the absence of pain receptors in the intestinal mucosa
The problem of delivering large molecules has led others back to injections and looking at the possibility of administering these inside the GI tract. “This is a painless process due to the absence of pain receptors in the intestinal mucosa,” says Vllasaliu. “[Microneedles] in particular [are] proving to be an exciting technology that is showing promising results.”
The most advanced device is from Rani Therapeutics, based in San Jose, California. In February 2019, the company announced it had successfully tested an unmedicated RaniPill capsule in humans, following more than 100 animal studies.
The coated RaniPill capsule is protected in the acidic stomach environment, but dissolves in the small intestine. Here, a reaction produces carbon dioxide which inflates a polymer balloon inside the capsule; this pushes a biodegradable microneedle filled with the drug into the intestinal wall.
“This technology has shown impressive insulin bioavailability,” says Vllasaliu. Rani says it has demonstrated insulin delivery that is at least on par with subcutaneous injections in animal studies — although the data supporting this has not been published — and the first human trial of the system without medication has been carried out.
A similar approach was taken by researchers at MIT, led by Langer and Traverso, when they developed the tortoise shell-shaped capsule. They recently published preclinical data on the ingestible self-orienting millimeter-scale applicator (SOMA) which injects insulin into the stomach wall[4]
. At 4–6mm thick, the stomach wall provides a larger protective layer and a more predictable and faster delivery time.
Traverso says the challenge was to find a method that “predictably engaged with the tissue every single time”. The resulting capsule’s centre of mass is lowered with high-density stainless steel so it always settles face down.
“Using such a small system in order to actually deliver a meaningful amount of drug as a liquid was going to be challenging,” says Traverso, so the team fabricated a millipost from the active pharmaceutical by pressing it into a solid — in this case a 7mm millipost of insulin. In the moist gastric environment, the sucrose coating melts away, releasing the spring-loaded millipost through the gastric mucosa. Results in rats and pigs have demonstrated that the SOMA can deliver insulin, and the solid drug is even more stable than standard formulations.
“Which therapeutics can be compressed into a solid and retain their activity is a question that will have to be experimentally derived,” Traverso adds.
He estimates that the research group will complete the first test in humans by 2023. But even this method will have some limitations around dosing, he suggests. “If we need to dose somewhere between 1–5mg per day, then this could serve that purpose. If we need to deliver a gram then this is not the route to deliver it.”
The future
Researchers are split over whether oral delivery could revolutionise the biologics market as a whole (see Box). Oral delivery “is not going to be the be-all and end-all,” predicts Shields.
“As biologics get larger, I believe they are going to continue to be delivered by injection.”
However, Mitragotri points out that there is tremendous interest from the pharmaceutical industry in seeing these oral technologies move forward. “Practically every major biotechnology company is using biologics for drug delivery to treat chronic diseases, so that’s where a simple way of delivering drugs, like orally, becomes very useful,” he says.
Just when oral insulin will make it into the hands of patients is difficult to predict. “It’s tough to put a timeline [on it] because there are so many steps that have to take place before [oral insulin] will be out,” says Mitragotri. “But, given the interest in the field and the new technology being introduced, I hope that it is sooner rather than later.”
Box: Oral delivery of monoclonal antibodies
There are currently more than 60 approved peptide drugs, but the majority of new biologics are monoclonal antibodies — large proteins with a molecular mass of above 150kDa — which severely limits the possibility of oral delivery. However, a few oral antibody drug candidates are being developed for gastrointestinal diseases, where the drug is intended to act locally.
Intract Pharma, a spin-off company from UCL School of Pharmacy, is pursuing a strategy that can deliver antibodies via the colon. “The large intestine is a more friendly environment,” says Vipul Yadav, director of research at Intract. “By delivering the same drug to the large intestines you can improve its bioavailability in the blood because you are bypassing some of the enzymes in the small intestine which limit absorption.”
Yadav adds that in addition to proteases, the small intestines also host efflux transporters — proteins that pump molecules back into the gut. There seems to be less of these efflux transporters in the colon, plus the transit time is longer (more than 24 hours), encouraging more drug uptake[5]
.
Intract’s Soteria delivery system starts by protecting the drug using its ‘Phloral’ technology — a pH-sensitive polymer and natural polysaccharide coating. “Only when the pill reaches the large intestines is the coating dissolved by the [higher] pH, as well as the presence of the microbiome. The bacteria that reside in the large intestines eat up the [polysaccharide] coating,” explains Yadav. This dual mechanism fail-safe ensures the drug is not released prematurely.
Inside the capsule, the formulation contains agents to protect the drug from enzymatic degradation while simultaneously enhancing uptake into the colonic tissue. In the case of specific gastrointestinal disease, the antibody drug is able to engage local targets. “High accumulation of the antibody in the gut allows it to enter into the tissue where it can utilise inflammation biomarkers, like tumour necrosis factor (TNF) — the major inflammatory pathways that are activated in diseases like Crohn’s [disease] and ulcerative colitis,” says Yadav.
The ability to deliver antibodies locally could have real clinical advantages, particularly given that long-term systemic delivery of antibody drugs has been linked to increased lymphoma risk[6]
. Intract is hoping to develop an oral formulation of an infliximab biosimilar, the TNF blocker used to treat autoimmune diseases and currently administered by intravenous infusion.
Although systemic delivery of oral biologics is a few years away, Yadav says that it could be revolutionary for monoclonal antibodies. “It was thought almost impossible to deliver [antibodies] orally a few years ago, and now for the past five years we’ve seen at least two companies taking on that challenge, so I think the future seems bright,” he says.
References
[1] Garg SK, Rewers AH & Akturk HK. Diabetes Technol Ther 2018;20(Suppl 2):S21–S24. doi: 10.1089/dia.2018.0101
[2] Whitehead K, Shen Z & Mitragotri S. J Control Release 2004;98(1):37–45. doi: 10.1016/j.jconrel.2004.04.013
[3] Pridgen EM, Alexis F, Kuo TT et al. Sci Trans Med 2013;5(213):213ra167. doi: 10.1126/scitranslmed.3007049
[4] Abramson A, Caffarel-Salvador E, Khang M et al. Science 2019:363(6427):611–615. doi: 10.1126/science.aau2277
[5] Ranmal, SR, Yadav V & Basit AW. Available at: https://www.intractpharma.com/wp-content/uploads/2018/10/Intract.pdf (accessed September 2019)
[6] Bongartz T, Sutton AJ, Sweeting MJ et al. JAMA 2006;295(19):2275–2285. doi: 10.1001/jama.295.19.2275
[7] Anselmo AC, Gokarn Y & Mitragotri S. Nat Rev Drug Discov 2019;18(1):19–40. doi: 10.1038/nrd.2018.183