BRUKINSA is the only BTK inhibitor recommended by NICE and accepted by SMC for adults with MZL who have received at least one prior anti-CD20-based therapy.1–5
MZL is an indolent B-cell lymphoma with heterogeneous clinical presentation6–8
The World Health Organization classifies MZL into three subtypes: extranodal, splenic, and nodal.6,7 MZL is linked to chronic inflammation and is associated with multiple infectious agents and autoimmune diseases.7,8 The heterogeneity of MZL can make treatment decisions challenging, needing a tailored approach based on the characteristics of the patient and disease subtype.7,8
While many patients achieve long remissions following initial therapy, relapse is common, especially among patients with advanced disease at diagnosis.6 Those who experience disease progression within 24 months of initial therapy have a median survival of only 3 to 5 years.7 Patients are typically older adults and many have co-morbidities leading to unacceptable toxicities with chemotherapy.6,7
BRUKINSA is a next-generation BTK inhibitor that has demonstrated efficacy in R/R MZL, across the subtypes.9 It is an oral, targeted treatment that has been well-tolerated in patients with R/R MZL and other B-cell malignancies across clinical studies.1,9
BRUKINSA is reimbursed and available for eligible patients in the UK3,4
SMC has announced that BRUKINSA is accepted for reimbursement within NHS Scotland, making it available to patients with R/R MZL across the UK.3,4
SMC acceptance for Scotland, December 2024
BRUKINSA monotherapy is accepted by SMC for the treatment of adult patients with marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based therapy.3
This advice applies only in the context of an approved NHS Scotland Patient Access Scheme (PAS) arrangement delivering the cost-effectiveness results upon which the decision was based, or a PAS/list price that is equivalent or lower.3
See more about BRUKINSA reimbursement in Scotland.
NICE recommendation for England and Wales, September 2024
The National Institute for Health and Care Excellence (NICE) recommends BRUKINSA as an option for treating marginal zone lymphoma in adults who have had at least 1 anti‑CD20-based treatment.4
It is only recommended if the company provides it according to the commercial arrangement.4
See more about BRUKINSA reimbursement in England and Wales.
BRUKINSA demonstrated deep and sustained responses across R/R MZL subtypes in the MAGNOLIA clinical study9
MAGNOLIA specifically evaluated BRUKINSA in patients with R/R MZL who have relatively limited treatment options6,9
MAGNOLIA was an open-label, single-arm Phase 2 study evaluating the efficacy and safety of BRUKINSA for the treatment of patients with R/R MZL who had received at least 1 anti-CD20–directed regimen. Eligible patients (N=68) were treated with BRUKINSA 160mg twice daily until disease progression, unacceptable toxicity, withdrawal of consent or the end of the study.9
MAGNOLIA evaluated a range of patients with R/R MZL, including patients with extranodal (n=26), nodal (n=26) and splenic (n=12) disease (4 patients had unknown subtype).1,9
The primary efficacy endpoint was IRC-assessed ORR, defined as the proportion of patients who achieved a best overall response of partial response or complete response. Secondary efficacy endpoints included investigator-assessed ORR, DoR, PFS and OS.9
BRUKINSA showed high response rates in R/R MZL, with a median response time of 2.8 months (range: 1.7, 11.1)1,9
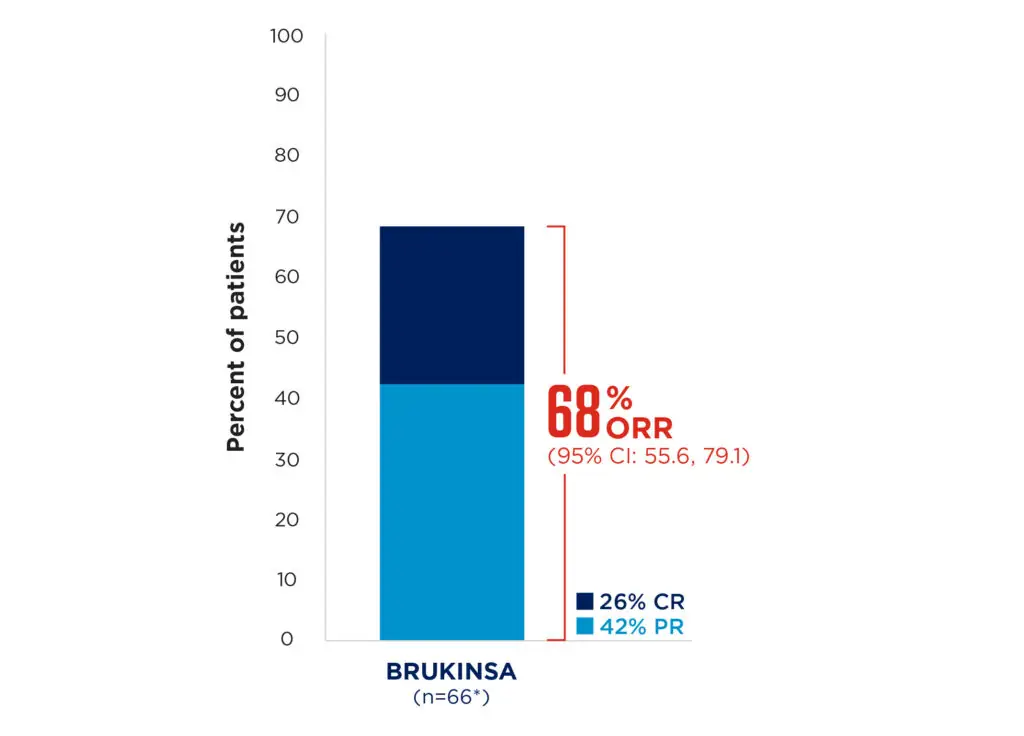
Median follow-up: 28.0 months
Adapted from Opat S, et al. 20239
BRUKINSA achieved deep and sustained responses, regardless of MZL subtype9
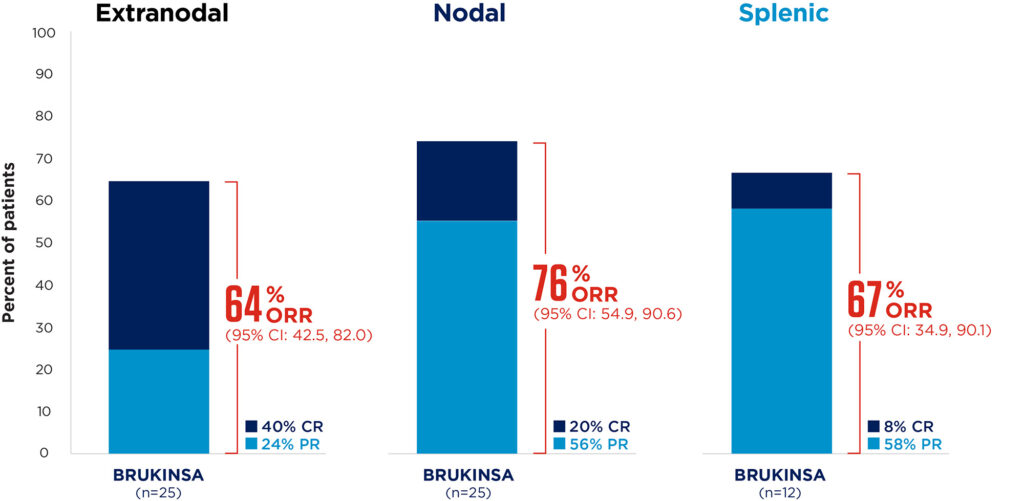
Median follow-up: 28.0 months
Adapted from Opat S, et al. 20239
Response rates were consistent across pre-specified subgroups, including patients traditionally considered difficult to treat, such as those with advanced age and advanced-stage MZL.9
BRUKINSA demonstrated durable disease control in R/R MZL9
At 24 months, the PFS rate was 70.9% (95% CI: 57.2, 81.0) and the DoR rate was 72.9% (95% CI: 54.4, 84.9). At the last analysis, patients were still responding well, with neither the median PFS or DoR reached (at a median follow-up of 27.4 and 23.4 months, respectively).9
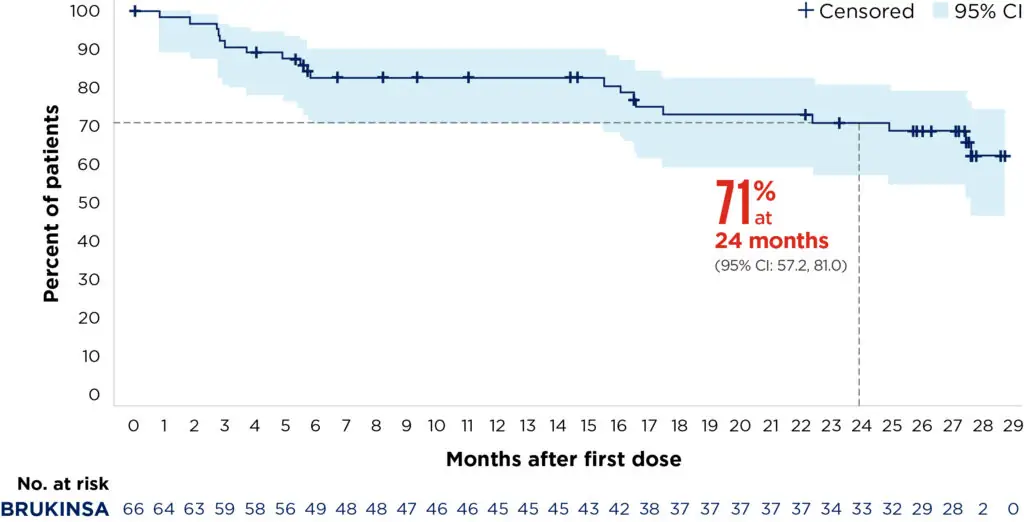
Median follow-up: 27.4 months
Adapted from Opat S, et al. 20239
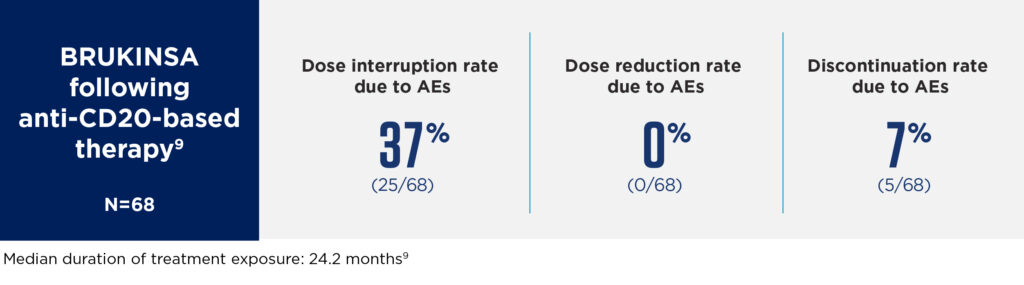
BRUKINSA was generally well tolerated, with no new safety signals observed†9
The most common Grade ≥3 AEs were neutropenia (8.8%) and COVID-19 (5.9%). BRUKINSA had low rates of AEs of special interest, including hypertension (any Grade: 4.4%; Grade ≥3: 2.9%) and atrial fibrillation/flutter (any Grade: 2.9%; Grade ≥3: 1.5%).† No cardiac AEs led to treatment discontinuation.9
Overall, BRUKINSA had a favourable safety profile in patients with R/R MZL, with low rates of dose interruptions, dose reductions and discontinuations due to AEs.9
Beigene
BRUKINSA’s favourable safety profile is well documented and consistent across indications1
In pooled safety data from 1550 patients with B-cell malignancies (median duration of treatment exposure: 34.41 months), the most commonly occurring adverse reactions (≥20%) were upper respiratory tract infection (36%), bruising (32%), haemorrhage/haematoma (30%), neutropenia (30%), musculoskeletal pain (27%), rash (25%), pneumonia (24%), diarrhoea (21%) and cough (21%).1
Of the 1550 patients treated with BRUKINSA, adverse reactions leading to dose reduction and treatment discontinuation occurred in 5.0% and 4.8% of patients, respectively. The most frequent adverse reaction leading to treatment discontinuation was pneumonia (2.6%).1
Refer to the BRUKINSA SmPC for complete safety information.
BRUKINSA is an oral therapy with flexible and convenient dosing1
- Treatment with BRUKINSA offers once-daily or twice-daily dosing options.1
- BRUKINSA can be co-administered with PPIs and H2RAs without compromising efficacy and can be dose-adjusted for patients who are taking moderate and strong CYP3A inhibitors.1
- No specific dose adjustment is required for elderly patients (≥65 years old) taking BRUKINSA.1
Refer to the BRUKINSA SmPC for complete information before initiating therapy with BRUKINSA.
BRUKINSA (zanubrutinib) as monotherapy is indicated for the treatment of adult patients with:1
- Chronic lymphocytic leukaemia (CLL)
- Waldenström’s macroglobulinaemia (WM) who have received at least one prior therapy, or in first-line treatment for patients unsuitable for chemoimmunotherapy
- Marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based therapy
Click here for BRUKINSA prescribing information
▼ This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Report any suspected adverse reactions.
Adverse events should be reported. United Kingdom (incl. Northern Ireland): Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme found at www.mhra.gov.uk/yellowcard. Ireland: Healthcare professionals are asked to report any suspected adverse reactions via HPRA. Adverse events should also be reported to BeiGene at adverse_events@beigene.com
Footnotes
*Two patients in MAGNOLIA (N=68) were not evaluated for efficacy due to central confirmation of MZL transformation to diffuse large B-cell lymphoma but were included for safety evaluation.1
†Five patients died due to AEs: COVID-19 pneumonia (n=2); myocardial infarction in a patient with preexisting cardiovascular disease (n=1); acute myeloid leukemia in a patient with prior exposure to an alkylating agent (n=1); septic encephalopathy following radical cystectomy and ileal conduit in a patient with recurrent bladder cancer (n=1).9
‡1 patient experienced atrial fibrillation and 1 patient experienced atrial flutter. Atrial fibrillation occurred 21 days after the last dose of BRUKINSA in a patient with a history of atrial fibrillation.9
Abbreviations
AE, adverse event; BTK, Bruton’s tyrosine kinase; CI, confidence interval; COVID-19, coronavirus disease 2019; CR, complete response; DoR, duration of response; H2RA, histamine H2-receptor antagonist; IRC, independent review committee; MZL, marginal zone lymphoma; NICE, National Institute for Health and Care Excellence; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PPI, proton-pump inhibitor; PR, partial response; R/R, relapsed or refractory; SMC, Scottish Medicines Consortium; SmPC, Summary of Product Characteristics.
1124-BRU-PRC-145 | December 2024
Cancer learning ‘hub’
Pharmacists are playing an increasingly important role in supporting patients with cancer, working within multidisciplinary teams and improving outcomes.
However, in a rapidly evolving field with numbers of new cancer medicines is increasing and the potential for adverse effects, it is now more important than ever for pharmacists to have a solid understanding of the principles of cancer biology, its diagnosis and approaches to treatment and prevention.
This new collection of cancer content, brought to you in partnership with BeOne Medicines, provides access to educational resources that support professional development for improved patient
- 1.BRUKINSA United Kingdom Summary of Product Characteristics. BeiGene UK, Ltd. 2024. Accessed December 2024. https://www.medicines.org.uk/emc/product/14001/smpc
- 2.Tam CS, Ou YC, Trotman J, Opat S. Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Review of Clinical Pharmacology. 2021;14(11):1329-1344. doi:10.1080/17512433.2021.1978288
- 3.SMC2684. Zanubrutinib (Brukinsa). Scottish Medicines Consortium. 2024. Accessed December 2024. https://scottishmedicines.org.uk/medicines-advice/zanubrutinib-brukinsa-full-smc2684
- 4.National Institute for Health and Care Excellence TA1001. Zanubrutinib for treating marginal zone lymphoma after anti-CD20-based treatment. National Institute for Health and Care Excellence. 2024. Accessed December 2024. https://www.nice.org.uk/guidance/ta1001
- 5.National Institute for Health and Care Excellence Health Technology Evaluation: Zanubrutinib for treating relapsed or refractory marginal zone lymphoma: Final scope. National Institute for Health and Care Excellence. 2024. Accessed December 2024. https://www.nice.org.uk/guidance/ta1001/documents/final-scope
- 6.Walewska R, Eyre TA, Barrington S, et al. Guideline for the diagnosis and management of marginal zone lymphomas: A British Society of Haematology Guideline. Br J Haematol. 2023;204(1):86-107. doi:10.1111/bjh.19064
- 7.Wang H, Wan X, Zhang Y, Guo J, Bai O. Advances in the treatment of relapsed/refractory marginal zone lymphoma. Front Oncol. 2024;14. doi:10.3389/fonc.2024.1327309
- 8.Cheah CY, Zucca E, Rossi D, Habermann TM. Marginal zone lymphoma: present status and future perspectives. haematol. 2022;107(1):35-43. doi:10.3324/haematol.2021.278755
- 9.Opat S, Tedeschi A, Hu B, et al. Safety and efficacy of zanubrutinib in relapsed/refractory marginal zone lymphoma: final analysis of the MAGNOLIA study. Blood Advances. 2023;7(22):6801-6811. doi:10.1182/bloodadvances.2023010668