Mark Watkinson
In July 2018, Billy Caldwell — a 12-year-old boy with severe epilepsy — was granted an exceptional licence by the Home Office to be treated with medical cannabis oil bought by his mother in Canada. The oil contains tetrahydrocannabinol, which is illegal in the UK, and had previously been confiscated, leading to a media furore.
Under review
Billy’s case led to the home secretary Sajid Javid announcing a two-part scheduling review on 19 June 2018 to consider the therapeutic evidence for cannabis and cannabis-related products and whether medical cannabis should be rescheduled.
Simultaneously, the government created a new expert panel, chaired by Sally Davies, chief medical officer for England and chief medical adviser to the UK government, to consider individual applications from senior clinicians wanting to prescribe cannabis-related medicinal products. Javid has made it clear, though, that the government has no plans to legalise cannabis for recreational use.

Source: Brian Lawless / PA Wire / PA Images
Billy Caldwell, with his mother, Charlotte Caldwell, who has campaigned for the law to be changed to allow her son to be treated with cannabis-derived medication
Cannabis is currently listed under Schedule 1 of the Misuse of Drugs Regulations 2001, meaning it is considered to have no known medicinal value and cannot legally be prescribed or otherwise procured for personal, including medicinal, use.
Placing cannabis under Schedule 2 instead of Schedule 1 would allow it to be prescribed and legally supplied by pharmacists. It would also enable research into the medicinal properties of cannabis, potentially leading to the wider use of cannabis-derived medicinal products.

Source: Charles Shearn / The Pharmaceutical Journal
Dame Sally Davies, the chief medical officer for England, led the first part of the government’s review into cannabis rescheduling, and has also been appointed to chair a panel of experts who will assess individual requests from clinicians to prescribe medical cannabis
The first part of the government’s cannabis scheduling review, which was also led by Davies, examined existing research into the therapeutic and medicinal benefits of cannabis-related medicinal products. Her report, which was published on 3 July 2018, recommends that cannabis-based medical products should be moved out of a Schedule 1 classification.
The publication of this report signalled the start of the second part of the review, led by the Advisory Council on the Misuse of Drugs (ACMD), which was asked by the Home Office to provide an assessment of which cannabis-related products, if any, should be rescheduled. It also recommended ending Schedule 1 restrictions on cannabis, but warned of the risks of inappropriate prescribing and called for a series of ‘checks and balances’ to be introduced.
Javid advised the House of Commons on 19 June 2018 that if this second review identified that cannabis offers significant medical benefits, “then we do intend to reschedule”.
At the time of publication, this decision had not been made, but it was expected imminently.
Industry support
The government’s new approach is unlikely to meet much opposition from the pharmacy profession. In June 2018, results from a Royal Pharmaceutical Society (RPS) survey showed that 80% of pharmacists agreed that cannabis should be legalised for medicinal use.
The three RPS national pharmacy boards have given their support to the rescheduling of cannabis to Schedule 2. In its signed policy statement that was sent to the Home Office for consideration on 20 June 2018, the RPS says the rescheduling would give “pharmaceutical scientists greater freedom to research its potential medicinal uses and to carry out clinical trials”.
Medical cannabis is reasonably safe and there is reasonable evidence of its efficacy
Clinicians and patient organisations also support the idea of rescheduling medical cannabis. Peter Carroll, director of campaigning organisation End Our Pain, says the move could have a “profound effect to the benefit of patients”.
“It is the most important step in the journey to securing legal access to medical cannabis under prescription,” he added.
Mike Barnes, consultant neurologist and consultant in rehabilitation medicine and honorary professor of neurological rehabilitation at Newcastle University, says the rescheduling of cannabis so it can be used in medicine “should definitely happen”.

Source: Courtesy of Mike Barnes
Consultant neurologist Mike Barnes believes that if the government goes ahead with rescheduling cannabis it will create a unique set of regulations around the drug’s availability
“It’s reasonably safe, and there is reasonable evidence of its efficacy,” he says.
In 2016, Barnes produced research for the all-party parliamentary group for drug policy reform that concluded that cannabis being placed under Schedule 1 was “inaccurate and misleading” and that the evidence firmly suggests “cannabis should be a legal product for medicinal use”.
His evidence is reinforced by Davies’s recent review findings. In her report, she says: “There is clear evidence from highly respected and trusted research institutions that some cannabis-based medicinal products have therapeutic benefits for some medical conditions.”
She cited a recent US review which found evidence for benefit from medical cannabis for a wide variety of conditions, such as chronic pain, multiple sclerosis, anxiety disorders and post-traumatic stress disorder (see pie charts).
However, Ash Soni, president of the RPS, says the lack of ability to carry out research under Schedule 1 means “we don’t know” about any possible adverse effects of medical cannabis. Instead, he says the main message to pharmacy about a change to Schedule 2 for medical cannabis is the importance of allowing “research to understand the benefits and risks”.
Experts say there is likely to be high demand for medical cannabis products from patients; End Our Pain estimates the rescheduling would result in around 1 million medical cannabis users in the UK. But Soni believes not all patients will be suitable for treatment.
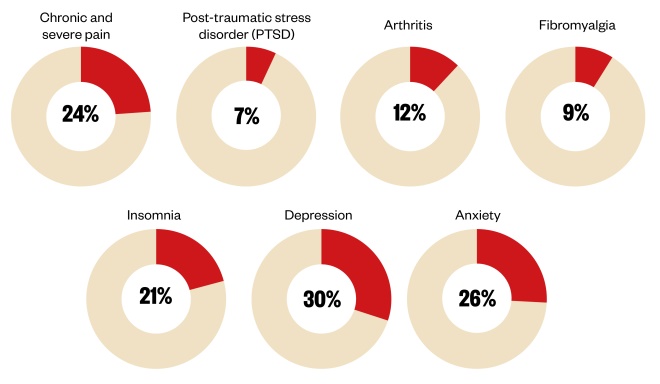
Figure: Cannabis use of the estimated 1 million medical cannibis users in the UK
Source: Online survey of medical cannabis use in the UK commissioned by the All-Party Parliamentary Group for drug policy reform from End Our Pain and United Patients Alliance.
He says patients requesting this treatment “have to see their doctor to say: ‘I need this for my pain’. And the doctor will need to explain that’s not the purpose [of medical cannabis] and it’s not [a] carte blanche to be prescribed under any circumstances, but will be available for specific individuals under specific conditions”.
If cannabis is moved to Schedule 2, it will set out who can supply and possess controlled drugs in a professional capacity and will lay down the conditions under which this can be carried out.
Schedule 2 drugs carry very strict prescribing rules and the regulations are clear that pharmacists must not dispense these drugs unless all information required by law is included on the prescription. Pharmacists can amend the prescription only for minor typographical errors and they must sign, date and include their General Pharmaceutical Council registration number when doing so.
Barnes says that in theory, once under Schedule 2, doctors would be able to prescribe cannabis-based drugs at their own risk, as with any other Schedule 2 medicine. But he believes cannabis may become a unique case.
“[The government] will want to restrict it for financial reasons if nothing else,” he says.
“I guess another panel will be formed or an advisory group formed that would determine how doctors prescribe, which doctors for which conditions. I would predict an advisory group would be the next stage.”
Answering patient queries
Whatever cannabis’s ultimate status, given pharmacy’s accessibility, it is likely to be on the frontline when facing patients’ general queries about the supply of cannabis for medicinal purposes.
The legality, safety and efficacy of cannabis oil and cannabidiol-containing products is still uncertain
A statement from the National Pharmacy Association (NPA) says pharmacy teams have seen “a considerable increase in requests for cannabis-related products from members of the public and we have been providing appropriate advice and guidance to NPA members in response”.
It says: “There are a number of legal, professional and ethical considerations around the sale/supply of cannabis oil and cannabidiol-containing products from community pharmacies.
“Additionally, the legality, safety and efficacy of cannabis oil and cannabidiol-containing products is still uncertain. No guidance within the UK is currently available.”
Learning from other countries
Pharmacists can look to other countries where medical cannabis is available legally to get a sense of what its impact could be in the UK. Medical cannabis is legally available on prescription in many European countries, including Germany, the Netherlands, Italy, Poland, Greece, Croatia and Finland.

Source: Shutterstock.com
Medical cannabis is legal in the Netherlands, as well as many other European countries
Israel legalised the products as long ago as 1994 and currently it has more than 30,000 users who obtain their medicines from pharmacies, with production regulated by the Israeli health ministry’s medical cannabis unit.
Germany legalised medical cannabis only in 2017, with products currently imported from Holland and Canada. Drugs are regulated by a cannabis agency working under the German Federal Institute for Drugs and Medical Devices and are available from pharmacies.
As well as learning from international experiences, some UK pharmacists may already have had direct experience of dispensing a cannabis-derived medicine in the same way as any other pharmaceutical product, as since 2010 they have been dispensing the MHRA-approved GW Pharmaceuticals medicine Sativex, which treats spasticity symptoms in adults with multiple sclerosis. This mucosal spray was made exempt from the Schedule 1 restrictions at the time — although it is not currently recommended by the National Institute for Health and Care Excellence for use on the NHS owing to its cost.
In June 2018, health minister Lord James O’Shaughnessy met with GW Pharmaceuticals to discuss the benefits of the company’s medicines and how the government can support the company through the Life Sciences Industrial Strategy.
The rescheduling of medicinal cannabis opens the door to accelerated research into the use of other drugs, such as MDMA and psilocybin
If rescheduled, the medical cannabis market is set to expand in the UK and more manufacturers of these products will start applying to the MHRA for a licence. But will this expansion be confined to cannabis or will other illegal drugs now be rescheduled and used for therapeutic uses?
Harry Shapiro, director of the charity DrugWise and a member of the ACMD, hopes the rescheduling of medical cannabis “opens the door to accelerated research into the use of other drugs, such as MDMA and psilocybin in the treatment of psychiatric disorders, bearing in mind that all such treatments have to pass muster the same as any other drug being brought to market”.
Meanwhile, a decision on the rescheduling of medical cannabis is imminent and many pharmacists are hoping for the drug to get the green light.
“It’s something we welcome. We’re not saying it should be available freely for people who perceive they need it. We’re saying we need further research into which elements of the drug are effective,” says Soni.
Vox pop: should cannabis be rescheduled?

Lila Thakerar, superintendent pharmacist, Shaftesbury Pharmacy, Harrow
“I’m in favour of medical cannabis being rescheduled as long as it’s monitored by the prescriber, such as the GP or hospital.
But the public needs to know that the cannabis available in the unlicensed home grown market is harmful, and is associated with a risk of addiction and mental health disorders.
I’m concerned that press reports and publicity that cannabis has moved to a safe license product that people will assuming you can buy unlicensed cannabis for recreational use.
The flood gates could open for people to want cannabis on demand for their health. The media needs to careful how it broadcasts the news of rescheduling. If it happens there needs to be cautionary guidelines and an update from relevant authorities such as the Home Office explaining the risks of using cannabis.
I don’t think rescheduling will change things drastically for pharmacy. Our role will be more signposting to relevant prescribers and sources of information.”

Terry Maguire, community pharmacist, Maguire Pharmacy, Belfast
“Cannabis should not be rescheduled. What we’re looking at is an agenda to internationally commercialise medical cannabis. This is only a stepping stone to abusing vulnerable children who are being used cynically by commercial companies. It’s only about the money.
There’s a massive fraud going on here. Children are being used as a means to get legal changes to a substance to try and encourage a route to [the use of] recreational cannabis.
The general public is being deliberately confused. There are so many different strengths and concentrations of cannabis so we can’t possibly be talking about a particular product when we’re talking about cannabis in a medicinal sense.”

Lindsey Fairbrother, owner and superintendent pharmacist, Goodlife Pharmacy; secretary of Shropshire local pharmaceutical committee; and practice pharmacist, Harley Street Medical practice, Stoke-on-Trent
“If there is a medical need then definitely ‘yes’ with a ‘but’. We have to make sure we have the evidence of the benefits, which I believe is there.
We have to make sure cannabis is correctly used for medical purposes, but we should be able to achieve that within the existing framework of scheduled controlled drugs legislation.
What we don’t want to be happening, which no doubt it is, are internet purchases of products that might not be pure and might not even be cannabis oil at all, since that could cause more harm.
Regulatory authorities need to take action to ensure that the issue of medical cannabis oil is regulated as any other controlled drug would be. But we should not let protracted legislation force patients to wait to receive the support they need.”