This content was published in 2006. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
The pharmacist has always been an essential member of the multidisciplinary nutrition support team, traditionally involved in providing technical advice on parenteral nutrition.1 However, pharmacist involvement in nutrition has also demonstrated a positive effect on clinical outcomes such as fluid balance2 and the transition from parenteral to enteral or oral feeding.3
There are many opportunities for hospital pharmacist involvement in aspects of nutritional care in the surgical patient — it is not just the remit of the nutrition team pharmacist. Every pharmacist can have a positive impact on patients’ nutritional status and assist in diet and therapy modification to minimise any decline in nutritional status during hospital stay.
Malnutrition is a common problem in perioperative patients, since the underlying disease may cause reduced nutrient intake(due to dysphagia, intestinal obstruction or anorexia) or malabsorption (due to chronic inflammation, biliary obstruction, pancreatic disease or intestinal fistulae). Nutrient requirements may be increased and intake is often decreased. Surgical management and drug therapy can also cause a decrease in nutrient intake, further compounding this problem.
Nutrition plays a key role in peri-operative care. Appropriate intervention in the pre-operative phase can positively affect post-operative outcome with a reduction in complications and length of stay.
The link between poor nutritional status and post-operative complications was first identified in the 1930s,4 and yet malnourished patients are still being admitted for elective surgery without appropriate intervention.
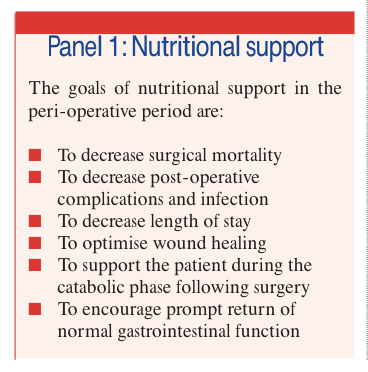
The goals of nutritional support in the perioperative period are listed in Panel 1.
Pre-operative optimisation
Pre-operative assessment clinics provide an ideal opportunity to improve nutritional status before a patient’s operation, and to minimise the peri-operative reduction in nutrient intake in elective surgical patients. A basic nutritional history, including details of weight, height, any history of unintentional weight loss or reduced intake, will identify any patients who are at risk. Steps can then be taken to optimise pre-operative nutritional status by referring appropriate patients for dietetic intervention.
The National Institute for Health and Clinical Excellence recommends that pre-operative feeding should be considered if a patient is malnourished, and that this should be provided via an enteral feeding tube inpatients with an unsafe swallow (ie, at risk of aspiration) or inadequate oral intake.5 This is also recommended by the American Society for Enteral and Parenteral Nutrition (ASPEN), which advises that pre-operative nutrition support should be administered for 7–14 days to moderately or severely mal-nourished patients.6 ASPEN also recommends that, if it is safe to do so, the operation should be delayed to allow this pre-operative nutrition support to be administered.
Early assessment of patients can also help to identify those who may be at high risk of post-operative nausea and vomiting (PONV) and allow for appropriate preventative therapy. An early assessment would also allow for the use of epidural anaesthesia and epidural analgesia to be considered.Using epidural anaesthesia and analgesia helps to reduce the incidence of post-operative ileus (gastrointestinal paralysis) and the metabolic response to injury by blocking visceral sympathetic and parasympathetic pathways.
The “Enhanced recovery after surgery”programme, pioneered in Denmark, is now beginning to have a higher profile in theUK.7This programme uses a multimodal approach to shorten the length of stay in hospital and improve outcome. The main focus is on the key indicators that dictate discharge date: post-operative pain, gut function and mobility.
Pre-operative fluid and carbohydrate loading, using a nutritional supplement, is given up to two hours before the operation.The patient starts taking oral fluids and feed on the night following the operation, with intravenous fluids being discontinued on the first or second post-operative day. Epidural anaesthesia and analgesia, regular non-opiate analgesia and prompt treatment of PONV all help to encourage early resumption of oral intake.This method has been successful in reducing length of stay for several patient groups, including those undergoing colorectal surgery.
Effect of drug therapy
Drug therapy can affect nutritional status in a number of ways: by reducing oral intake, altering nutrient absorption or affecting nutrient metabolism.8 Taking a detailed drug history and recording basic nutritional para-meters will help identify patients at risk of drug-nutrient interactions. As would be expected, there is a directly proportional, linear relationship between the number of drugs a patient is taking and the number of drug-nutrient reactions for which the patient is at risk.9
Gastrointestinal disturbances are the most commonly reported side effect of drug therapy10 so it is not surprising that drug therapy can affect nutritional intake.
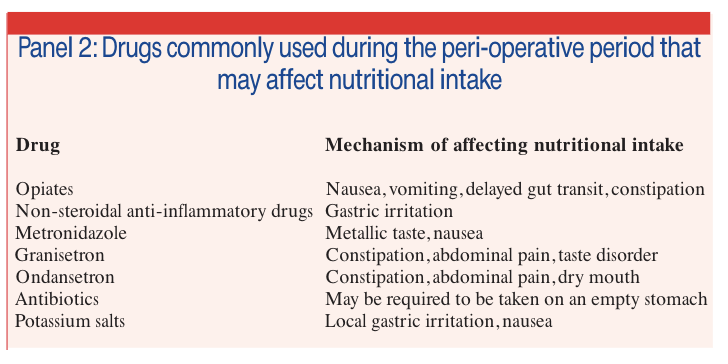
There are many drugs used during the peri-operative period that may affect nutritional intake. Some of these are described in Panel 2.
Peri-operative requirements
Patient’s nutritional requirements may change during the peri-operative period. Energy requirements are based on age, sex and body weight (with adjustments in obese patients). Standardised “stress factors” help tocalculate increased needs as a result of tissue repair and the effects of systemic inflammation. These stress factors are dependent on the operative procedure and the clinical condition of the patient. When considering protein (or amino acid) provision, increased requirements for wound healing and increased metabolic activity should be taken into account.
Any surgical procedure will elicit a metabolic response. Hormonal and inflammatory mediators increase the mobilisation and turnover of fatty acids, amino acids and their precursors from skeletal muscle and adipose tissue. This provides the substrates for the functioning of essential organs and for tissue repair.
The size and duration of metabolic response is determined by the complexity and length of the procedure. A minor, uncomplicated operation produces a transient increase in nutritional requirements that is unlikely to be clinically important. However, a major, protracted procedure will elicit a significant metabolic response and increase nutritional requirements. Consideration should also be given to any pre-existing inflammation that may increase nutritional requirements, such as active inflammatory bowel disease.
Serum albumin concentrations fall rapidly post-operatively, primarily due to haemodilution, extravascular loss and decreased synthesis. For these reasons, albumin is not considered a useful marker of nutritional status in acutely ill patients.
Routes of administration
The route of administration of nutrition support, is influenced by a number of factors, particularly in the post-operative period.
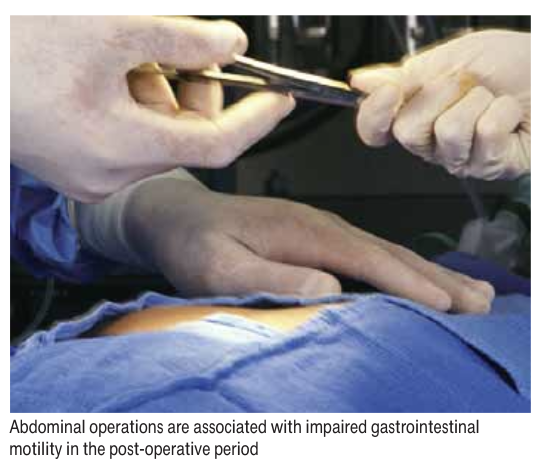
Historically, patients were kept “nil by mouth” post-operatively until they had passed flatus. All abdominal operations, including laparascopic surgery, and major procedures outside the peritoneal cavity are associated with impaired gastrointestinal motility in the early post-operative period. This is compounded by drugs which affect motility, such as opiates and 5HT3-receptor antagonists. Barium studies have demonstrated that colonic motility is the last gastrointestinal function to return, taking up to three to five days, whereas small bowel motility returns within 24–48 hours.11 However, these studies were carried out in the early 1970s, and since surgical, anaesthetic and analgesic techniques have progressed much since then, it is likely that these durations will now be shorter.
Providing the patient has a safe swallow and there are no specific concerns about gut function or integrity, oral nutrition support should be provided within 24 hours of surgery. If the swallow is unsafe, nutrition support should be provided via an enteral feeding tube within 48 hours of the operation. There is no evidence that early introduction of post-operative oral nutrition increases complication rates.
Oral nutrition support can be provided as food or as sip feeds and supplements. The type of surgery may influence the choice of food or supplement.
Enteral feeding tube access is increasingly being used electively to deliver nutrition during the peri-operative period. Pre-operatively placed gastrostomy tubes are particularly useful for patients undergoing head and neck surgery, and intraoperatively placed jejunostomy tubes are useful for oesophagogastric and hepatobiliary surgery. If enteral access is not secured for these patients parenteral nutrition becomes necessary.
Nasogastric tube feeding can also be useful in the peri-operative period where a temporary increase in nutritional intake is required. When there is poor tolerance to enteral nutrition, NICE recommends the use of motility agents such as metoclopramide or erythromycin to encourage gastric emptying.
Enteral tubes and drugs
In some surgical patients the only route of enteral access available is the feeding tube which is used for both nutrition and drug therapy. Pharmacists can play an important role in the optimisation of enteral nutrition through their input into medicines management of patients whose enteral feed-ing tube is used both for the administration of medicines and enteral nutrition, and through the use of medicines to improve tolerance to enteral nutrition. It is essential that drug therapy administered via an enter-al feeding tube is effective and does not compromise nutritional intake through interaction or tube blockage.
There are some important drug administration issues for patients with enteral feeding tubes. Finding a suitable drug formulation for administration to the surgical patient with limited gastrointestinal accessor dysphagia can be difficult. Although par-enteral administration can be used (and often guarantees 100 per cent absorption),repeated intravenous, subcutaneous or intra-muscular injections are associated with complications and are not suitable for continuous long-term use. There are also other routes that can be considered, such as trans-dermal, buccal, rectal or topical, but the drugs available in these preparations are limited. In these patients the feeding tube is often the only means of enteral access and is increasingly being used as a route for drug administration.
A systematic approach can be taken to find the most appropriate therapy by considering each of the questions below in turn.
Is this drug needed at all? A thorough medicines review should always be the first step in the decision process. Unnecessary medicines should be stopped.
Can the drug be administered via any other route? For short-term therapy it maybe appropriate to use injections or suppositories. For longer-term therapy a transdermal patch, depot injection or nasal spray may be more appropriate.
Does the drug come in a suitable formulation for administering via a tube? Tube blockage and gastrointestinal side effects are the two main factors that affect formulation choice. It is known that nurses who seek advice from a pharmacist are more likely to administer the correct formulation of a medicine via the feeding tube. In this case there is also a lower incidence of tube blockage.12,13 Suitable formulations for administration via a tube are considered to be:
- Solutions
- Syrups
- Suspensions
- Soluble tablets
- Effervescent tablets
- Dispersible tablets
However, factors such as sorbitol content, osmolarity, sodium content and physical composition (eg, a granular suspension)need to be taken into account.
If there is no suitable formulation, canI crush the tablet or open the capsule? Crushing tablets and opening capsules should be considered to be a last resort because of the risks to the person administering the medicine. Crushing tablets in open containers such as mortars or medicine pots, or opening capsules to obtain the powder contained within, will increase the risks of inhalation by the operator potentially leading to sensitisation, allergies, absorption and possible adverse effects.
Under the Health and Safety Commission’s Control of Substances Hazardous to Health regulations every employer must provide employees with information, instruction and training to ensure that the employee knows the risks created by expo-sure to substances hazardous to health and the precautions that should be taken.
Medicines such as corticosteroids, hormones, antibiotics, immunosuppressants, cytotoxics and phenothiazines are irritant or very potent and extra precautions should betaken when handing these medicines.
There are many slow-release preparations that are marketed for their once-daily convenience. Crushing these tablets and administering them via enteral feeding tubes can have fatal consequences when the entire daily dose is administered as a bolus.14 Crushing tablets in order to administer them via a feeding tube also increases the incidence of tube blockage and increases the risks of adverse effects.Consideration should be given to changing to an alternative drug that is available in an appropriate formulation for administration via a feeding tube.
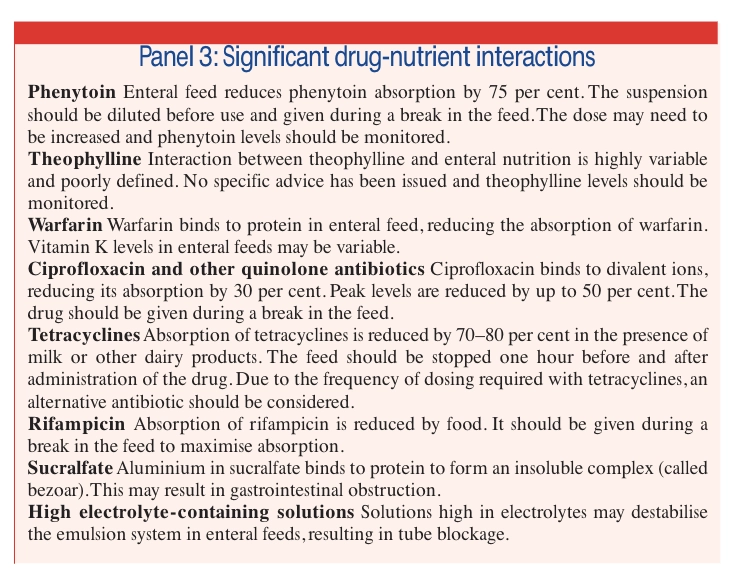
Does it interact with the enteral feed? There are three types of interaction between enteral feeds and drugs. First, the feed can either decrease or increase the absorption of the drug. Second, there may be a physical interaction between the drug and the feed, usually resulting in tube blockage. Third, the action of the drug, usually its pharmacological action, can affect the function of a nutrient. Significant drug-nutrient interactions are outlined in Panel 3.
For most drugs there are no specific data on the interaction with enteral feed and therefore clinical monitoring is essential.
Is the drug absorbed from where it is being delivered to?
The site of exit of the feeding tube has implications for drug delivery and subsequent absorption. The tube may terminate beyond the drug’s main site of absorption or result in higher peak levels due to rapid jejunal absorption.
Can the drug be given safely? There have been a number of significant drug errors,15–17including one reported death,18 associated with enteral drugs being administered via the wrong route, due to the inappropriate use of injectable syringes for the measurement and administration of these drugs. The continued reporting of similar incidents highlights the need to put systems in place to prevent this. The National Patient Safety Agency will shortly be issuing guidance on this topic.
Parenteral nutrition
Parenteral nutrition used to be the mainstay of post-operative nutrition support. However, a trend towards more aggressive use of enteral nutrition and the realisation that patients should not be kept “nil by mouth” for post-operative bowel rest have seen a decline in the use of parenteral nutrition in these patients.
Despite the increased use of enteral nutrition, there will always be patients who require parenteral nutrition. The most common post-operative indications for parenteral nutrition are an ileus, a perforation, or the formation of a fistula.
NICE recommends that parenteral nutrition is only indicated when oral or enteral intake is inadequate or if the patient has anon-functional, inaccessible or perforated gastrointestinal tract. It also recommends that parenteral nutrition should be considered for the peri-operative period in malnourished patients unable to meet their nutritional requirements via other routes.
If the patient does not already have suit-able central access for parenteral nutrition,NICE recommends that peripheral administration should be considered for treatment courses of less than two weeks.
Where possible, parenteral nutrition should meet a patient’s nutritional (both macro- and micro-nutrient), fluid and electrolyte requirements.This will reduce the need for additional fluids and electrolytes, requiring less venous access and manipulations.
Fluid and electrolyte balance can be com-plicated in the immediate post-operative phase due to sodium and fluid retention. Inpatients without excessive losses a moderate amount of sodium should be included.19 However, patients with high nasogastric or stoma losses will have high requirements for electrolytes.
An accurate nutritional history should betaken before starting parenteral nutrition to identify patients at risk of refeeding syndrome (which can result in fluid and electrolyte shifts when nutrition support is started). This can be a particular problem for patients with a poor pre-operative nutritional status and a prolonged post-operative period with no nutritional intake.
NICE makes clear recommendations on the management of nutrition support inpatients at risk of refeeding syndrome. In general it also recommends that parenteral nutrition should be started slowly (at 50 percent of estimated requirements) for the first24–48 hours.
Parenteral nutrition therapy is best man-aged by a multidisciplinary team, in which the pharmacist is a key member.
Summary
Effective patient monitoring is an essential aspect of any clinical pharmacy service. In addition to monitoring the drug therapy, pharmacists can have an active role in monitoring nutritional progress, helping nurses to identify patients who have absent or poor oral intake and ensuring appropriate dietetic referrals are made when necessary.
Recent legislation provides more opportunities for pharmacist involvement through supplementary prescribing. NICE guide-lines recommend that each organisation should have a nutrition steering group, which should include a pharmacist. This group can have a positive impact on organisational guidelines relating to pre-operative fasting and nutritional screening.

References
- Allwood MC, Hardy G, Sizer T. Roles and functions of the pharmacist in the nutrition support team.Nutrition 1996;12:63–4.
- Broyles JE, Brown RO, Vere KL, Nolly RG, Lugher RW. Pharmacist interventions improve fluid balance in fluid-restricted patients requiring parenteral nutrition. Drug Intelligence and Clinical Pharmacy1991;25:119–22.
- McDermott LA, Christensen VD. Nutritional support: pharmacists’ influence on the prescribing process.Topics in Hospital Pharmacy Management 1994;14:30–9.
- Studley HO. Percentage of weight loss: a basic indicator of surgical risk in patients with chronic peptic ulcer. JAMA 1936;106:358–60.
- National Collaborating Centre for Acute Care.Nutrition support in adults. Oral nutrition support, enteral tube feeding and parenteral nutrition.London: The Centre;2006.
- American Society of Parenteral and Enteral Nutrition board of directors. Guidelines for the use of parenteral and enteral nutrition in adult and paediatric patients: perioperative nutrition support. Journal of Parenteral and Enteral Nutrition 2002; 26(S):95SA–96SA.
- Fearon KCH, Luff R. The nutritional management of surgical patients: enhanced recovery after surgery.Proceedings of the Nutrition Society2003;62:807–11.
- White R, Ashworth A. How drug therapy can affect, threaten and compromise nutritional status.Journal of Human Nutrition and Dietetics2000;13:119–29.
- Lewis CW, Frongillo EA, Roe DA. Drug-nutrient interactions in three long-term-care facilities. Journal of the American Dietetic Association 1995;95:309–15.
- Bateman DN. Gastrointestinal disorders. In: DaviesDM (editor). Textbook of adverse drug reactions. 4th edition. Oxford: Oxford Medical Publications; 1991.pp230–44.
- Nachlas MM, Younis MT, Roda CP, Wityk JJ.Gastrointestinal motility studies as a guide to postoperative management. Annals of Surgery1972;175:510–22.
- Seifert CF, Frye JL, Belknap DC, Anderson DC. A nursing survey to determine the characteristics of medication administration through enteral feeding catheters. Clinical Nursing Research 1995;4:290–305.
- Belknap DC, Seifert CF, Petermann M. Administration of medicines through enteral feeding catheters. American Journal of Critical Care1997;6:382–92.
- Schier JG, Howland MA, Hoffmann RS, Nelson RS.Fatality from administration of labetalol and crushed extended-release nifedipine. Annals of Pharmacotherapy 2003;37:1420–3.
- Cousins D, Upton D. More wrong route problems.Pharmacy in Practice 1999; 9:18–19.
- Cousins D, Upton D. Wrong route is used yet again.Pharmacy in Practice 2000;10:63.
- Cousins D, Upton D. Oral paracetamol liquid administered intravenously. Pharmacy in Practice2001;11:221.
- Cousins D, Upton D. Inappropriate syringe use leads to fatalities. Pharmacy in Practice 1998;8:209–10.
- White R, Bradnam V. Drug administration via enteral feeding tubes. London: Pharmaceutical Press;2006.
- Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 2002;359:1812–18.