Shutterstock.com
When Rebecca Klaper searched for signs of pharmaceuticals in Lake Michigan, she got a surprise. The most common drug she found was one she hadn’t even considered looking for — metformin, a diabetes drug.
“It wasn’t even on our radar,” says Klaper, a freshwater scientist at the University of Wisconsin-Milwaukee in the United States. “We only found it because the Environmental Protection Agency just happened to add it to the detection assay we used.”
Perhaps even more surprising was how far the drug had spread from the point it entered the lake via treated sewage. “Lake Michigan is huge, so we expected a big dilution effect, but we were still finding drugs, including metformin, three miles from the sewage treatment plants,” she says.
Klaper was also interested in what effect the drug might be having on fish in the lake. Metformin is used to treat diabetes, so she looked at the metabolism of fish exposed to a similar concentration of metformin in the laboratory, but saw no metabolic changes. Instead, she found that a gene related to egg production was being expressed in male fish, which indicates hormonal changes. She concluded that metformin could be having a feminising effect on male fish, and may decrease their ability to reproduce.
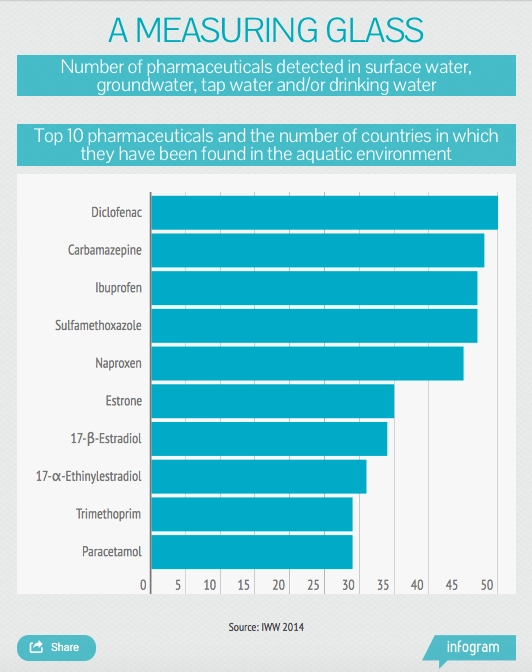
Lake Michigan is far from being an isolated case. A 2014 global review of pharmaceuticals in the environment, commissioned by Germany’s environment ministry, found that of the 713 pharmaceuticals tested for, 631 were found above their detection limits. They are found all over the world — in 71 countries across all of the United Nations’ five regional groups[1]. They were found mainly in surface waters, such as lakes and rivers, but also in groundwater, soil, manure and even drinking water.
Scientists are studying the effect of these drugs on ecosystems, and are trying to find ways of preventing the problem, for example by the correct disposal of unwanted medicines, improving the treatment of sewage and, ultimately, designing more environmentally friendly drugs.
It’s a real, growing problem, and it’s only going to get worse as the world’s population ages
“It’s a real, growing problem, and it’s only going to get worse as the world’s population ages,” says Gwynne Lyons, policy director of the CHEM Trust, a UK environmental charity.
We probably have an incomplete picture of the problem, however, because we don’t have detection methods for all of the thousands of pharmaceuticals in use around the world, and the analytical methods are not standardised internationally, so detection limits may vary, Lyons adds.
But some drugs are worse than others because of their potential to affect wildlife or people. They include antibiotics, antidepressants, anti-inflammatories and analgesics, beta-blockers, oral contraceptives and hormone replacement therapies.
Drug delivery
There are three main ways that pharmaceuticals make their way into the environment. By far the biggest contribution comes from drugs taken by people or animals that are then excreted in urine or faeces.
“A good proportion of any drug is excreted,” says Lyons — between 30% and 90% of the active ingredient in an oral dose. And the metabolites of many drugs can also remain active in the environment after being excreted.
The improper disposal of drugs also makes a contribution — when people fail to complete a prescription or clean out their medicine cabinet and throw the leftover drugs in the sink or down the toilet. In both cases, drugs end up in sewage treatment plants, which were generally not designed to remove such pollutants from wastewater. Depending on the drug, removal efficiencies range from 20% to more than 80%.
A 2014 report by UK Water Industry Research found that in most of 160 sewage treatment works studied, several common drugs were present in the final effluent in concentrations high enough to potentially affect ecosystems[2]. The drugs included the anti-inflammatories ibuprofen and diclofenac, the antibiotics erythromycin and oxytetracycline, and the female sex hormone 17b-estradiol.
Some drug manufacturing facilities have also been shown to release active ingredients into nearby waterways, creating localised hotspots of pharmaceutical pollution. In 2009, Joakim Larsson, an environmental pharmacologist from the University of Gothenburg in Sweden, found high concentrations of antibiotics downstream of several drug manufacturing facilities near Hyderabad in India — in some cases the levels were equivalent to doses given therapeutically[3]. Two years later, Larsson found high levels of known antibiotic resistance genes in the bacteria there.
Population crash
In most cases, however, the concentrations of pharmaceuticals found in the environment are much lower than the therapeutic dose. In rivers and lakes that receive treated wastewater, drugs are typically found in concentrations of 0.1 mg/L to 1.0 mg/L. Klaper found concentrations of metformin in Lake Michigan of around 40 mg/L close to the sewage treatment plants, but only 0.012mg/L offshore.
Because of these low concentrations, it can be difficult to determine what, if any, effect the drugs are having on the ecosystem. Much of the time the affected organisms are not in the public eye, for example algae or sea lice, or the effects are mild enough to go unnoticed if researchers aren’t specifically looking for them. “We generally don’t have the degree of surveillance we need until there is a population crash in a larger animal,” says Lyons.
Such a population crash occurred between 1996 and 2007, when millions of vultures in India were killed by exposure to the anti-inflammatory drug diclofenac, driving the birds to near-extinction1. The drug was given to cattle to treat pain and fever, and because people in India do not eat beef, the carcasses of dead cattle were left for the vultures to feast on, including cattle recently given high doses of diclofenac. It turns out that vultures from the genus Gyps are particularly sensitive to diclofenac, and between 10 million and 40 million birds died from abdominal gout and acute kidney failure. Three species of Gyps vultures are now critically endangered in Asia. Similar toxic effects have been seen in Gyps vultures in Africa and Europe, although without the same population decline.
But such dramatic and clear-cut links between pharmaceutical exposure and harmful ecological effects are rare. Indeed, the case of diclofenac and vultures is the only one in which the environmental impact in the wild has been solely attributed to a drug, according to Jason Snape, principal environmental scientist at pharmaceutical company AstraZeneca.
Other ecotoxicological effects of drugs in the environment have been found — the veterinary anti-parasite drug ivermectin, for example, has been found to reduce the number and variety of insects in cow dung, which can delay dung degradation and potentially reduce the amount of food available to birds and bats[4]. But the consequences are generally milder, and the cause-and-effect relationship is less clear. And few drugs have been studied outside laboratory experiments.
Laboratory studies have found that anti-depressants can alter the spawning behaviour of clams, disrupt movement in snails, cause altered aggressive behaviour in crayfish, and affect learning in cuttlefish4. The anti-inflammatory drug ibuprofen has been reported to affect reproduction in fish, including the delayed hatching of eggs, in the laboratory4.
Feminised fish
One of the human pharmaceuticals whose environmental effects have been studied extensively is ethinylestradiol, the active ingredient in many contraceptive pills. It has been shown to affect the sexual development of male fish at extremely low concentrations in the laboratory, and intersex fish have been found downstream from sewage plants in rivers around the world. A seven-year, whole-lake experiment in Canada found that chronic, low-level exposure to the drug could affect both male and female fish, and led to a near-extinction of the study population, although the concentration in the experiment was higher than that generally seen in rivers[5].
But John Sumpter, an ecotoxicologist at Brunel University in London, says that the evidence that estrogen from contraceptive pills is feminising male fish in the wild is not conclusive, and there is no evidence for population-level effects. “The concentrations required for a population crash are much higher, sometimes by several orders of magnitude, than those seen in rivers,” he says.
The issue is complicated by the fact that the same feminising effects can result from exposure to many other hormone-like chemicals that might be present in the water, including bisphenol A, that have been shown to have endocrine-disrupting properties.
“Pharmaceuticals in the environment is a very active field, and there have been many papers recently claiming effects at environmentally relevant concentrations,” says Sumpter. “But I have serious questions about the quality of many of those papers.”
Sumpter recently led a large, EU-funded project called PHARMAS on the effects of pharmaceuticals in the environment. It focused on anticancer drugs because little was known about how they might affect ecosystems. In a rare bit of good news in this field, Sumpter and his colleagues found no serious effects at the low concentrations typically seen in the environment. “Anticancer drugs do not appear to be of environmental concern,” he says.
Both Sumpter and Lyons agree that much more research needs to be done to determine what drugs are out there and which ones are causing problems in the real world. “There is a real lack of field studies,” says Lyons.
Cocktail party
In particular, there is a need to study the ‘cocktail effect’ — the way different drugs can interact to produce adverse effects. Pharmacists and doctors warn patients that they should not mix ibuprofen with beta-blockers, but a fish swimming in a soupy mixture of drugs and other chemicals in a polluted river doesn’t have that option.
Multiple kinds of similar drugs could also have an additive effect, says Sumpter. “If we have six different beta-blockers, maybe we should do the risk assessment on their combined concentration,” he says.
One forthcoming study could help to answer these questions. The Otter Project at Cardiff University has received funding to screen for pharmaceuticals in the carcasses of otters from around the UK. Traces of diclofenac and ibuprofen have previously been found in otters, says Elizabeth Chadwick, the project’s director, and the team is now considering which other chemicals and drugs to look for.
Otters are a good species to use for such a study, says Chadwick, because they are relatively long-lived and are at the top of the food chain, which gives the drugs time to accumulate in their system. “Chemicals in water can be very transient, so water testing is hit and miss,” she says. “Studying biota means they are captured in situ.”
The project will also allow Chadwick to consider potential drug interactions. “Ecological research looks at the whole cocktail,” she says.
Chadwick isn’t sure what effects she will find in the otters, but she is expecting to see some feminising effects, such as a reduction in sperm count, as has been found in fish. “There is a diverse range of potential health effects,” she says. “Until we have good data, we won’t know which ones are happening.”
Growing concern
The issue of pharmaceuticals in the environment is gaining more attention around the world. The Strategic Approach to International Chemicals Management (SAICM), an initiative run by the United Nations Environment Programme that aims to foster the responsible use of chemicals, is considering whether to list environmentally persistent pharmaceutical pollutants as an emerging policy issue at its next meeting in September 2015.
The European Union has included three drugs — diclofenac, 17a-ethinylestradiol (used in oral contraceptives) and 17b-estradiol (used in hormone replacement therapy) — on a ‘watch list’ for further study. The EU already requires companies to conduct an environmental risk assessment (ERA) for all new drugs, but it applies only to those authorised after 30 October 2005. As a result, says AstraZeneca’s Snape, only about 500–600 of the roughly 3,000 drugs in use in Europe have a full ERA with data on the chronic effects.
Once those assessments are completed, they can quickly become dated and may not reflect the latest science, adds Snape. “From an environmental perspective, there is no requirement for anything like the pharmacovigilance that goes on after a drug is approved,” he says. “You do your ERA, then once the marketing approval is given, you put it in a drawer.”
AstraZeneca, however, has developed a system of ‘ecopharmacovigilance’, in which it tracks new data on the environmental effects of its drugs so it can keep risk assessments up to date. “It protects the environment, but also our reputation,” says Snape.
But even if an ERA finds a high risk of environmental harm, it cannot prevent the marketing of a drug — and few would want it to. The needs of humans should not come second to environmental concerns, says Lyons. “But we need to look at the best option to reduce the risk.”
In some cases that might mean ‘source control’ — reducing the quantity of pharmaceuticals that enter sewage systems. This can be done by educating people about the possible environmental effects of what they stock in their medicine cabinet, and encouraging them to return unused drugs to the pharmacy for proper disposal. But Snape says this goal is hampered by the tendency of television dramas to show people flushing pills down the toilet.
In Sweden, drugs are graded on their environmental effects, and doctors are required to prescribe a less damaging drug where the option exists. Lyons would like to see this system rolled out more widely.
A more long-term solution might lie in the concept of ‘benign by design’, in which drugs are designed from the start to be less harmful to the environment. Many drugs include molecules that are not found in biology — such as halogen groups — that make them more persistent in the body and the environment. Sumpter wants environmental scientists to be involved much earlier in the drug discovery process to advise on how to balance efficacy with environmental concerns. “Before we go and tack another fluorine on the molecule, we should ask, are there other ways?” he says.
Snape says that the pharmaceutical industry is moving in that direction. Several companies have invested in a project under the EU’s Innovative Medicines Initiative to develop tools to screen environmental properties earlier in drug development. The increasing importance of biologic drugs, which break down more readily, will also help. But it will be a long process. “A lot of the drugs that will be entering the market five years from now have already been discovered,” Snape says.
In many cases, for the foreseeable future the only option will be to improve sewage treatment to reduce the amount of drugs that reaches rivers and lakes. But that will be expensive. Lyons wants the drug industry to bear much of the cost in a ‘polluter pays’ scheme. “The pharmaceutical companies should take more responsibility for their products over their life cycle,” she says.
Klaper agrees that more care needs to be taken to keep pharmaceuticals out of the environment as much as possible. She says: “In most cases, we don’t even know what they are doing to the ecosystem or to people.”
References
[1] Weber F-A, aus der Beek T, Bergmann et al. Pharmaceuticals in the Environment — the Global Perspective (IWW, 2014).
[2] Risk Based Prioritisation of Pharmaceuticals (UK Water Industry Research, 2014).
[3] Carlsson G, Orn S & Larsson DGJ Effluent from bulk drug production is toxic to aquatic vertebrates. Environ. Toxicol. Chem. 2009;28:2656–2662.
[4] Lyons, G. Pharmaceuticals in the Environment: A Growing Threat to our Tap Water and Wildlife (CHEM Trust, 2014).
[5] Kidd KA, Blanchfield PJ, Mills KH et al. Collapse of a fish population after exposure to a synthetic estrogen. Proceedings of the National Academy of Sciences USA 2007; 104:8897–8901.