Science Photo Library
Concerns about OTC ibuprofen could come from some of the guidance seen for prescription doses of the drug, and an assumption that the same applies at OTC levels, but is this caution supported by the evidence?
What is the safety profile of ibuprofen?
You can inform patients by explaining some of the facts around ibuprofen safety:
FACT: A large-scale clinical trial showed that reported GI adverse events (AEs) are very similar between OTC ibuprofen and paracetamol[2]
(see figure 1).
FACT: Ibuprofen has a low GI risk compared with other NSAIDs[3],[4]
.
FACT: An endoscopy study showed that the degree of muscosal damage seen with ibuprofen is generally dose-dependant, with little or no mucosal injury seen with 1200mg/day.[5]
OTC ibuprofen is as well tolerated as paracetamol on the GI tract*[2]
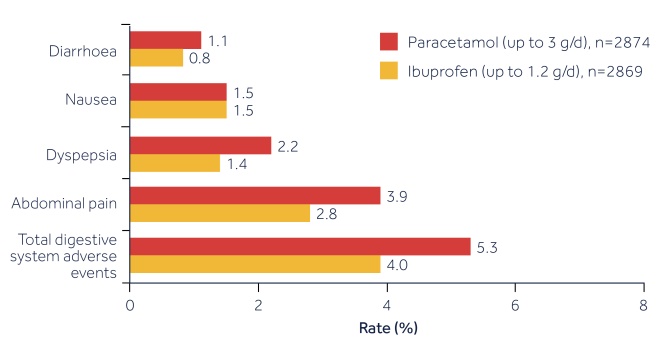
Figure 1: Gastrointestinal adverse events: rates of most frequent significant adverse events by COSTART body system and terms
Source: Adapted from Moore et al. 1999.[2]
*There are some special warnings and contraindications regarding GI safety of ibuprofen (including those with pre-existing GI conditions) – please see SPCs for full details.
*Based on the evaluable patient number.
OTC dose ibuprofen has a lower risk of GI events than prescription dose ibuprofen[6]
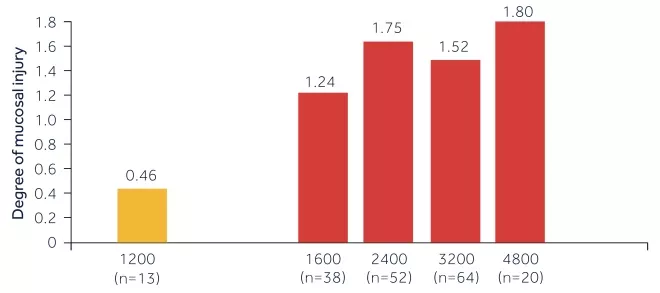
Figure 2: Gastric mucosal injury in people taking ibuprofen (n=187)
Source: Adapted from Lanza et al. 1984
[6]
FACT: There is no evidence that food has a gastroprotective benefit when taking OTC ibuprofen[7]
. Food may delay NSAID absorption[7],[8],
[9]
. In suitable patients, it may be more appropriate to take NSAIDs when fasting.
Download the resource here.
Essential information
Nurofen Express Liquid Capsules (Ibuprofen 400mg). For symptomatic relief of non-serious arthritic conditions, rheumatic or muscular pain, backache, neuralgia, migraine, headaches, dental pain, dysmenorrhoea, feverishness, colds and influenza. License Holder: Reckitt Benckiser Healthcare (UK) Ltd, SL1 4AQ. Legal category: P. Information about this product, including adverse reactions, precautions, contra-indications, and method of use can be found at https://www.medicines.org.uk/emc/product/5632.
To explore the evidence on ibuprofen safety and efficacy in more detail, please visit rbupdates.co.uk/ibuprofen
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Reckitt Benckiser Healthcare (UK) ltd on: 0333 200 5345
VEEVA CODE: RB-M-13382
Date of preparation: August 2020
References
[1] McCaul F et al. SelfCare 2019;10(3):79–92.
[2] Moore N et al. Clin Drug Invest 1999;18:89–98.
[3] Hersh EV, Moore PA & Ross GL. Over-the-counter analgesics and antipyretics: a critical assessment. Clin Ther 2000;22(5):500–548.
[4] Henry D, Drew A & Beuzeville S. Chapter 8: Gastrointestinal adverse drug reactions attributed to ibuprofen. In: Rainsford KD (ed). Ibuprofen: A Critical Bibliographic Review. CRC Press, Taylor and Francis: London; 1999.
[5] Bjarnason I. J R Soc Med 2007;100 Suppl 48:11–14.
[6] Lanza FL. Am J Med, 1984;77:19–24.
[7] Rainsford KD & Bjarnason I. J Pharm Pharmacol 2012;64: 465–469.
[8] Geisslinger G et al. Int J Clin Pharmacol Ther Toxicol 1989;27:324–328.
[9] Moore RA et al. Cochrane Database of Systematic Reviews 2015;11:CD010794. doi: 10.1002/14651858.CD010794.pub2



