Shutterstock.com / JL
Whooping cough (pertussis), a childhood disease of the past, is making a comeback. According to the US Centers for Disease Control and Prevention, there was an epidemic of more than 48,000 cases in the United States in 2012 — more cases than in any year since 1955 (see Figure 1). Data from Public Health England (PHE) show that, in the same year, almost 10,000 cases were diagnosed in England, causing 14 infant deaths — a startling ten-fold rise from levels in the previous decade (see Figure 2).
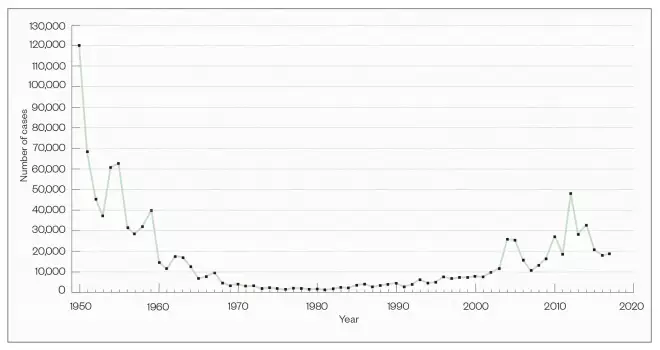
Figure 1: Reported cases of whooping cough in the United States
There was an epidemic of more than 48,000 pertussis cases in the United States in 2012
Source: US Centers for Disease Control and Prevention
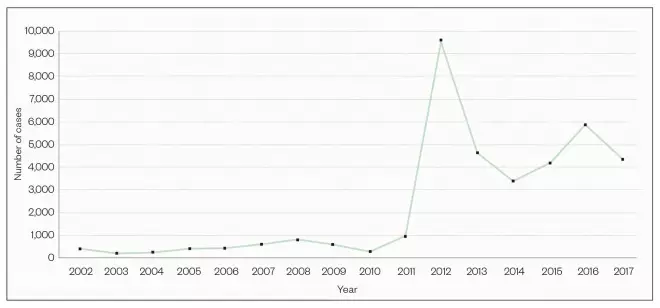
Figure 2: Confirmed cases of whooping cough in England
Almost 10,000 cases of whooping cough were diagnosed in England in 2012 — a ten-fold rise from levels in the previous decade
Source: Public Health England
At first glance, these epidemics may seem like the result of low vaccination rates, perhaps owing to anti-vaccination sentiments, but pertussis vaccination rates remain high in both the UK and the United States. Epidemiologists and other scientists have been investigating why incidence of the disease is on the rise in some countries and what interventions are needed to stop it.
Whooping cough is so named because of the severe coughing fits it causes and the high-pitched ‘whoop’ sound that can occur on inhaling at the end of a coughing episode. Coughing is so severe it can lead to vomiting, fatigue and, in some cases, even broken ribs. US statistics indicate that almost 10% of recorded cases of whooping cough result in admission to hospital, with the majority of deaths (0.12% of cases) occurring in children aged less than one year[1]
. The illness is caused by the bacterium Bordetella pertussis, with symptoms owing to several toxins secreted by the bacterium, including pertussis toxin.
Pertussis has persisted in less developed parts of the world and still represents the most prevalent vaccine-preventable childhood disease, with an estimated 160,000 deaths in children aged under five years globally[2]
. But the increases in incidence in the UK, the United States and Australia have not been seen in all countries — something different is going on in these developed countries and it appears to be connected to changes in the type of vaccine used.
Whole cell to acellular
Before the first vaccination was introduced in the 1950s, Health Protection Agency data show more than 100,000 suspected cases of pertussis in England and Wales per year. In some years more than 2,000 people died. By the 1970s, vaccination had reduced prevalence to 1,000–2,000 cases annually.
In the UK, the pertussis vaccine is given to infants aged two, three and four months, as part of the six-in-one vaccine (DTaP/IPV/Hib/HepB) that protects against diphtheria, tetanus, polio, Haemophilus influenzae type B and hepatitis B, as well as pertussis. A pre-school booster is given at around four years of age.

Source: Gayatri Amirthalingam
Gayatri Amirthalingam, consultant epidemiologist at Public Health England, says coverage of the whooping cough vaccine in the UK remains high
The current pertussis vaccine is acellular: it contains only a few selected pertussis antigens. It was introduced in the UK in 2004, replacing the previous whole-cell vaccine that contained the whole microorganism, killed through a physical or chemical process.
Unsubstantiated fears that the whole-cell vaccine caused more serious side effects, such as brain damage, led to a collapse in coverage in the 1970s and 1980s in the UK; but by 1992, coverage had reached 92% in England and Wales[3]
. “The coverage increased quite dramatically in the late 1980s and, since that time, our coverage has been consistently above 90%,” says Gayatri Amirthalingam, consultant epidemiologist at Public Health England. The switch to the acellular vaccine stemmed largely from availability issues — the required combination vaccine used the acellular version.
The coverage increased quite dramatically in the late 1980s and since that time our coverage has been consistently above 90%
In the United States, however, the move to the acellular vaccine in 1996 was fuelled by increasing parental unease with the whole-cell vaccine. ‘‘It was known to have inflammatory side effects, including local injection site reactions, fevers and occasionally seizures, so there was a push against it, to move towards the acellular vaccine,” explains Keith Rubin, founder and chief executive of vaccine company ILiAD Biotechnologies, based in New York.

Source: Keith Rubin
Keith Rubin, founder and chief executive of vaccine company ILiAD Biotechnologies, says that the current whooping cough vaccines don’t induce potent mucosal immunity
By the 2000s, most of Europe had also switched to the acellular vaccine. But the cheaper whole-cell vaccine is still used in middle- and low-income countries, and analysis of pertussis levels seems to indicate that epidemics are not being experienced in these countries. This suggests that the resurgence in the UK could be linked to the new acellular vaccine.
Asymptomatic infection
In 2015, epidemiologist Ben Althouse, now at the Institute for Disease Modelling in Bellevue, Washington, concluded that the acellular vaccine protected from symptomatic disease, but did not stop infection or transmission of pertussis — known as asymptomatic transmission[4]
. “Asymptomatic transmission basically means that individuals have the bacteria in their nose or in their lungs but they don’t know it,” explains Althouse. “They can still transmit that bacterium to another individual and potentially make them sick if they are not vaccinated.”
Althouse reached this conclusion by modelling epidemiological data from UK and US pertussis cases. Epidemics of pertussis tend to peak every four years. The reason for this is not fully understood, but it is thought to result from cycling in population immunity[5]
. Althouse found the UK and US data showed similar age-specific rates of pertussis, with peaks in four-year cycles. But in each country, the peaks were shifted to reflect the date at which the acellular vaccine was introduced — 1996 in the United States and 2004 in the UK. Althouse believes this is linked to increased rates of asymptomatic transmission occurring when acellular-vaccinated children reach school age and start to become infected and transmit the disease.
“Those two similar patterns in two different populations give us evidence that the mechanism is working the same way,” he says.
Further evidence for the asymptomatic transmission theory comes from the genetic variation found in the B. pertussis pathogen. The genomes of 150 B. pertussis samples predicted more genetic diversity in the overall bacterial population than would be expected from the number of pertussis infections and the average mutation rate. “That indicates that there is a lot more transmission; that there are a lot more bacteria floating around in the population,” Althouse says.
You vaccinate everyone around them but the babies are still getting sick at the same rate that they were before
Another indicator is the failure of ‘cocooning’, whereby all individuals in close contact with infants too young to receive the vaccine are vaccinated in an attempt to prevent transmission. “In fact, this does not work,” says Althouse. “You vaccinate everyone around them but the babies are still getting sick at the same rate that they were before.” The model created by Althouse and his team suggests that only 30–50% of transmission causes the disease, with the rest being asymptomatic.
Tod Merkel, a microbiologist at the US Food and Drug Administration in Bethesda, Maryland, has investigated the spread of pertussis using infant baboons, who develop similar symptoms to infected humans[6]
. Baboons were immunised with either the whole-cell or acellular vaccines and exposed to pertussis at the age of six to nine months. “What we found was that both vaccines protect against the disease very well: there are absolutely no outward signs of illness,” says Merkel.
But there was a difference in vaccinated animals compared with animals previously infected naturally. With natural immunity, it was not possible to reinfect the animals: ‘’We would see no signs of disease and it wasn’t possible to recover bacteria out of those animals.”
However, Merkel notes that there was a “startling” difference in acellular-vaccinated animals: “After they were infected, they became colonised and remained colonised longer than unvaccinated animals. Although they weren’t sick, they were carrying the bacterium in their airways.” When these animals were co-housed, they were able to infect other, unvaccinated, animals.
A similar pattern was seen with whole-cell vaccinated animals, but they were able to clear the infection within two weeks — much quicker than the acellular-vaccinated animals. “To a large extent, this can explain the increase we are seeing in cases of pertussis,” says Merkel.
Wrong sort of immunity
It seems that, although both vaccines induce robust antibody responses, the acellular vaccine induces the wrong type of cell-mediated immunity. “Current pertussis vaccines don’t induce potent mucosal immunity,” says Rubin. “So in some populations with over 90% vaccination rates you still have a large reservoir of people walking around with Bordetella pertussis in their nose and nasal passages.”
In some populations with over 90% vaccination rates you still have a large reservoir of people walking around with Bordetella pertussis in their nose and nasal passages
The mucosal immune system provides a separate defence against infection, uniquely adapted to protect the mucosal surfaces of the respiratory tract, nasal passages and intestines by physically preventing bacteria and viruses from attaching to mucosal surfaces.
An effective vaccine stimulates the development of T-cells, which provide an immune memory that protects against future reinfection. The T-cells reproduce and mount a stronger immune response when reinfection occurs, recruiting other cells to help kill the bacteria. The immune system produces different types of these T-cells, including the T-helper cells TH1 and TH2, which generate responses against intracellular and extracellular infections, respectively. But there is also a TH17 lineage, which controls bacterial infections specifically at mucosal surfaces.
Merkel studied these T-cells in baboons previously infected with B. pertussis and found they produced strong TH1 and TH17 responses. But, he explains, “when you vaccinate with the acellular vaccine, what we see is a very strong TH2 response, which is why we think people are protected from [symptomatic] disease”. But you see virtually no TH1 or TH17 cells: “It’s inducing the wrong type of immunity to clear the infection.” The study suggests either a strong TH17 or TH1 response may be necessary to kill and clear the bacteria from the airway, and that is what you see in a natural infection, and with the whole-cell vaccine, he explains.
Retaining herd immunity
If asymptomatic transmission is occurring, it calls into question whether herd immunity, normally accompanying high levels of vaccination, is providing protection for unvaccinated individuals. Althouse believes it is not, although Merkel is more optimistic: “I think it’s wrong to say that there is no herd immunity,” he says, suggesting that asymptomatic transmission is likely to be at a lower rate than transmission via symptomatic patients who will be coughing. “It’s important for people to understand that the acellular vaccine does protect against disease, especially in an era where there is a lot of anti-vaccine sentiment. And by reducing disease it reduces transmission.”
But some new measures are needed to deal with the pertussis resurgence. What about returning to the whole-cell vaccine, which is still used in many countries? Althouse has suggested that a way forward could be to vaccinate infants with a priming dose of the whole-cell vaccine. “A cohort of children born in 1996, right around when we were swapping from the whole cell to the acellular vaccine [in the United States], got one or two doses of the old vaccine and then finished with the new [acellular] vaccine. They had much lower rates of disease,” he explains. His team modelled this strategy and concluded it could save lives by reducing incidence of pertussis by up to 95%[7]
.
However, Merkel points out that the side effects associated with the whole-cell vaccine are still problematic. “We are not confident that parents and the population would be accepting of a vaccine that’s reactogenic in that way. They weren’t in the 1970s and 1980s; I don’t think there is any reason to think they would be now.” Althouse admits that it is unlikely to be a successful strategy, but adds “it would be an interesting idea and potentially even with the side effects, it could save lives”.
Since 2012, the UK has pioneered maternal vaccination as a way of reducing infections in infants[8]
. Mothers vaccinated at least one week prior to delivery seem to pass on their immunity to vulnerable newborns. “It’s been remarkably effective, offering well over 90% protection against pertussis for babies in the first two months of life, when we know the risks can be particularly high as they haven’t received their own vaccines,” says Amirthalingam. The programme in England has achieved relatively high coverage — around 72% of pregnant women were vaccinated in 2017/2018.
New vaccines
However, it is clear that new, more effective, vaccines are needed. There are already several in development: the most advanced candidate is ILiAD Biotechnologies’ live attenuated pertussis vaccine, BPZE1, which was developed in the laboratory of Camille Locht at the Institut Pasteur, Lille, France.
An attenuated vaccine is an altered version of the live pathogen with substantially reduced virulence. Locht achieved this by genetically engineering the bacteria to eliminate, attenuate or inactivate three harmful toxins. “The goal has been to deliver the kind of potent mucosal and systemic immunity induced by a natural infection, but without the toxicity,” explains Rubin. The new vaccine is administered intranasally, which he suggests helps to give a more natural and potent immunological response.
ILiAD’s baboon study showed that BPZE1 reduces nasal colonisation by 99.8% compared with the current acellular vaccine, and 99.5% compared with the whole-cell vaccine[9]
. “These are not subtle differences — this is ‘night and day’ with respect to the impact of a potent mucosal response,” Rubin says. BPZE1 seems to stimulate the local mucosal memory T-cell response that does not occur with the other vaccines.
These are not subtle differences — this is night and day with respect to the impact of a potent mucosal response
ILiAD is now conducting a phase II clinical trial in the United States and, so far, the vaccine has shown a clean safety profile with none of the side effects seen with the whole-cell vaccine. It hopes the vaccine could be available by 2023 as an adolescent and adult vaccine to replace current boosters. But, Rubin says, “we have done preclinical work that suggests the vaccine may also be effective as a neonatal vaccine. If we could vaccinate infants shortly after birth, before they leave the hospital, it would address the vast majority of infant mortality that occurs. That really would be a holy grail.”

Source: Lisa Morici
Lisa Morici, a microbiologist and associate professor at Tulane University, is working on an adjuvant for acellular whooping cough vaccine that could bolster its effectiveness
Another pertussis vaccine is being developed at Tulane University in New Orleans, Louisiana. “Our approach is not necessarily to replace the acellular pertussis vaccine, but to improve it by adding what is known as an adjuvant,” explains Lisa Morici, microbiologist and associate professor at Tulane University. An adjuvant is an additional substance delivered with a vaccine that enhances the long-term immune response. “What we need is to restore the T-cell, and in particular the TH17 cell, response that the whole-cell vaccine was able to enlist. We think we can do that by adding an adjuvant,” says Morici.
The alum adjuvant currently used in many vaccines does not stimulate the correct T-cell response, so Morici and James McLachlan, an immunologist at Tulane University, have been using outer-membrane vesicles (OMVs). These are tiny lipid nanoparticles that are shed from bacterial membranes. “The host immune system has evolved to recognise these tiny vesicles as a sign of life because dead things don’t shed vesicles — only living things,” says Morici. “They are very small and very potent at low doses, and so we can still illicit a robust immune response without causing any sort of harmful side effects.”
OMVs are an emerging concept in the vaccine field. They are currently used in the meningococcal group B vaccine Bexsero, which was approved by the European Medicines Agency in 2013. With funding from the US National Institutes of Health, Morici and McLachlan are undertaking animal studies on an intranasal pertussis vaccine containing OMV derived from the soil bacteria Burkholderia
spp.; however, it is likely to be another six to eight years before their vaccine is ready for clinical trials.
Mapping out the future
Morici believes that understanding the inherent weakness of the acellular pertussis vaccine and improving on it could be the springboard for a new approach to vaccines. “I think acellular pertussis is just the tip of the iceberg,” she says. “If we can prove the concept with these vesicles in this particular formulation, then we can move on to other diseases.”
As with the acellular pertussis vaccine, most vaccines stimulate antibody production that protects from the disease. But most pathogens that lack a vaccine, including those that cause malaria, tuberculosis and HIV, require the T-cell component — the wider immune response that, according to Morici, OMVs are able to elicit. “It really could be used in next-generation vaccines to protect against so many more diseases,” she says.
Rubin believes that BPZE1 could eradicate pertussis altogether. He compares its potential impact with the introduction of a new haemophilus influenzae type b (Hib) vaccine in the early 1990s. Prior to this, Hib infections were a serious health problem, but the new vaccine made infections rare. “It induced potent mucosal immunity and, within ten years, there was a 99% reduction in haemophilus influenza infections of that kind,” says Rubin. “It was an extraordinary accomplishment and we are hoping to do for pertussis what that vaccine did for [Hib].”
In the short term, UK efforts are geared towards improving the rates of maternal vaccination by ensuring pregnant women are offered the vaccine at the optimal time (at between 20 weeks and 32 weeks). The public health message also needs to keep explaining the continued importance of the pertussis vaccine and the direct protection it offers for infants in particular. “I think of vaccines as bullet-proof vests,” says Merkel. “They don’t protect you completely but you are better off with them than without. If we are correct about what’s going on, there is more circulation of pertussis in the population [than we think], so it’s even more important that you be vaccinated.”
References
[1] Skoff TH, Hadler S & Hariri S. The epidemiology of nationally reported pertussis in the United States, 2000–2016. Clin Infect Dis 2018;ciy757. doi: 10.1093/cid/ciy757
[2] Yeung KHT, Duclos P, Nelson EAS & Hutubessy RCW. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis 2017 17(9):974–980. doi: 10.1016/S1473-3099(17)30390-0
[3] Amirthalingam G, Gupta S & Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill 2013;18(38). PMID: 24084340
[4] Althouse BM & Scarpino SV. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med 2015;13:146. doi: 10.1186/s12916-015-0382-8
[5] Guris D, Strebel PM, Bardenheier B et al. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clin Infect Dis 1999;28(6):1230–1237. doi: 10.1086/514776
[6] Warfel JM, Zimmerman LI &Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. PNAS 2014;111(2):787–792. doi: https://doi.org/10.1073/pnas.1314688110
[7] DeAngelis H, Scarpino SV, Fitzpatrick MC et al. Epidemiological and economic effects of priming with the whole-cell Bordetella pertussis vaccine. JAMA Pediatr 2016;170(5):459–465. doi: 10.1001/jamapediatrics.2016.0047
[8] Choi YH, Campbell H, Amirthalingam G et al. Investigating the pertussis resurgence in England and Wales, and options for future control. BMC Med 2016;14(1):121. doi: 10.1186/s12916-016-0665-8
[9] Locht C, Papin JF, Lecher S et al. Live attenuated Pertussis vaccine BPZE1 protects baboons against Bordetella pertussis disease and infection. Journ Infect Dis 2017;216(1):117–124. doi: 10.1093/infdis/jix254