You can view the full infographic here
Cannabis plants contain more than 100 cannabinoids, as well as other compounds like flavonoids and terpenoids, which is thought to be why some people respond better to herbal cannabis than pure cannabis derivatives. Cannabinoids act on the body’s endocannabinoid system, which helps regulate many bodily functions via CB1 receptors, found mainly in the brain; CB2 receptors in immune cells; the gastrointestinal tract; and peripheral nervous system. The two most studied cannabinoids are THC and CBD.
THC (δ-9 tetrahydrocannabinol)
THC is the psychoactive component of cannabis. Weak partial agonist on CB1 and CB2 receptors. THC potency in dried cannabis has increased from an average of 3% in the 1980s to around 15% today. Some strains contain more than 25% potency
CBD (cannabidiol)
CBD does not produce psychoactive adverse effects. Has little affinity for CB1 and CB2 receptors directly. It is believed to modulate the effects of THC throughout the endocannabinoid system which is why the THC/CBD ratio can lead to different responses and adverse effects
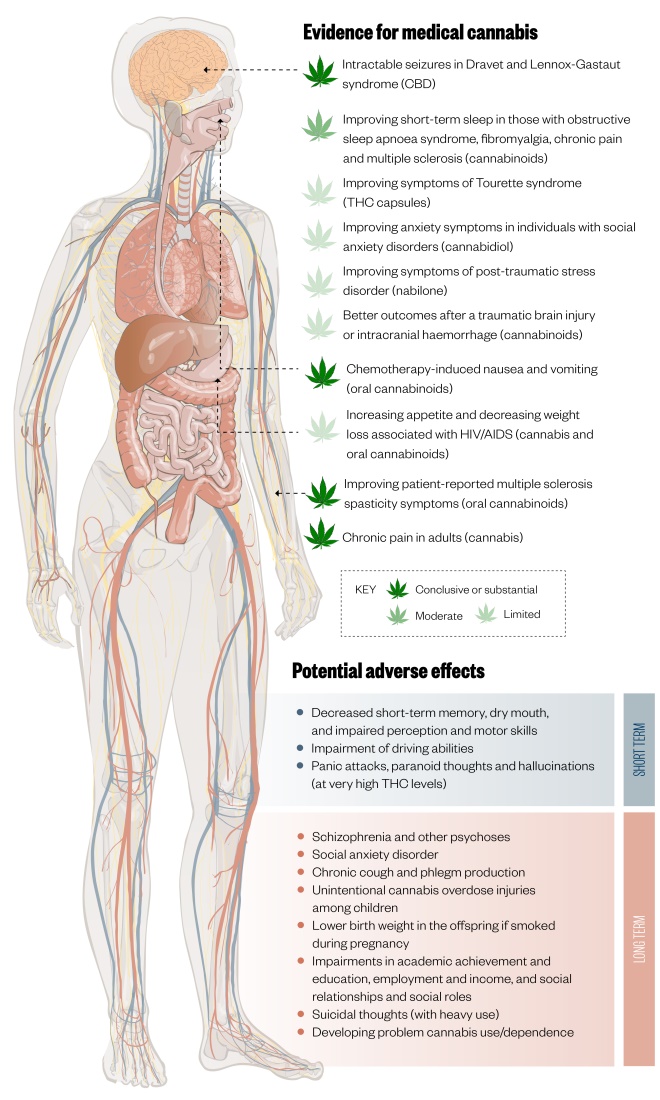
Figure 1: Evidence for medical cannabis and potential adverse effects
There is conclusive or substantial evidence for the use of medical cannabis in treating intractable seizures in Dravet and Lennox-Gastaut syndrome, chemotherapy-induced nausea and vomiting, improving symptoms in multiple sclerosis and treating chronic pain in adults, however the evidence regarding other indications is moderate or limited and there are several potential side effects.
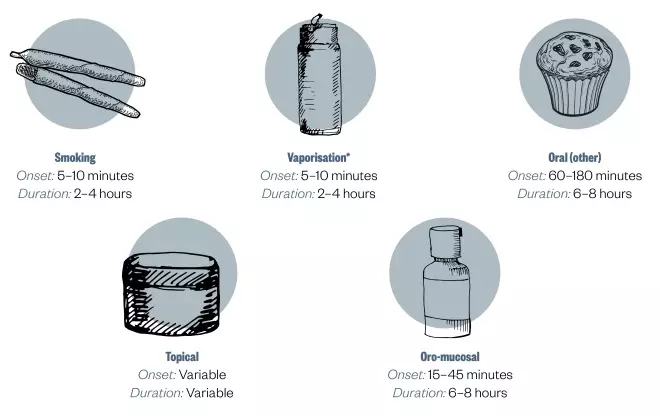
Figure 2: Routes of cannabis administration and dosing
*using a vaporising device that blows hot air through finely ground cannabis at a specified temperature
There are no established uniform dosing schedules for products such as fresh marijuana, smoked/vaporised marijuana or cannabis oil. Patients should start with a very low dose, e.g. 1mg THC, and titrate slowly until the desired effect is achieved, stopping if unacceptable adverse effects occur.
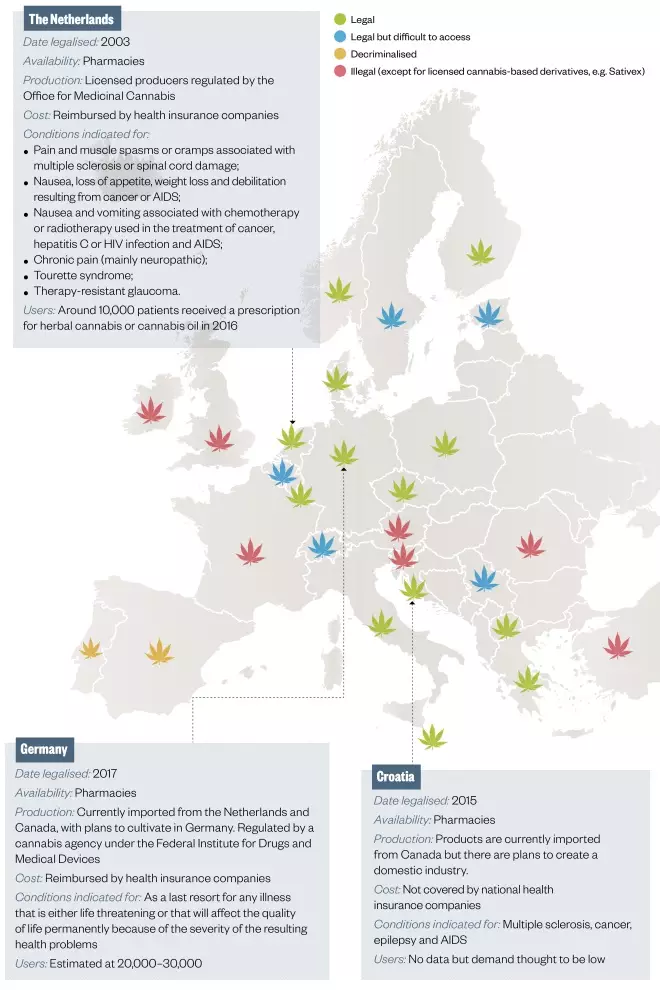
Figure 3: Medical cannabis regulation in Europe
Different models of regulation exist. In most countries, the government controls cannabis production and supply is restricted to regulated outlets (e.g. pharmacies), although some also allow self-cultivation. Some countries restrict the conditions for which cannabis can be prescribed.
References
Sources: Stockings E, Zagic D, Campbell G et al. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry 2018;89:741–753; National Academies of Sciences, Engineering and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. 2017; The National Academies Press, Washington DC; MacCallum CA & Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med 2018;49:12–19; Prohibition Partners. The European cannabis report. 3rd edition. 2018; Prohibition Partners, London.
Editorial adviser: Michael Boivin, pharmacist consultant, CommPharm Consulting Inc., Canada.
Infographic: MAG


