Viral challenge
There are three types of influenza viruses – A, B and C – but only types A and B cause seasonal epidemics in humans. Type A viruses can also cause pandemics and are classified into subtypes according to the variety and combinations of two surface proteins, hemagglutinin (HA) and neuraminidase (NA). Circulating flu viruses continuously evolve, so seasonal vaccines contain a mix of two influenza A subtypes and one or two influenza B viruses, which are reviewed each year by the World Health Organization (WHO).
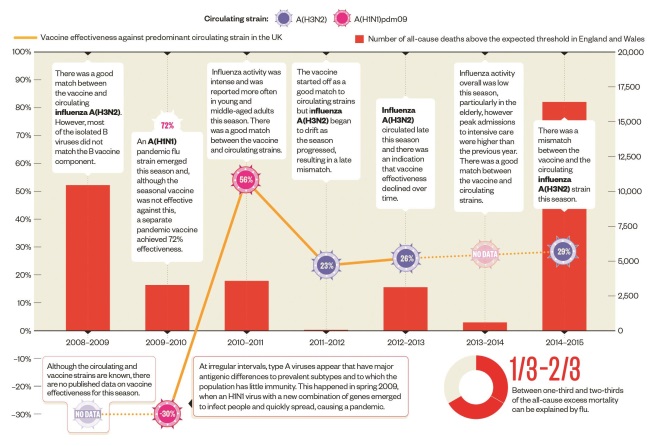
Vaccine effectiveness
The WHO coordinates a network called the Global Influenza Surveillance and Response System (GISRS), consisting of 142 National Influenza Centres (NICs) as well as various collaborating centres and laboratories. Recommendations for vaccine composition are made by the WHO in February for the following winter’s flu season in the Northern hemisphere. Antigenic drift of circulating viruses can occur in the interim, leading to a mismatch between the vaccine and circulating strains, and low vaccine effectiveness.
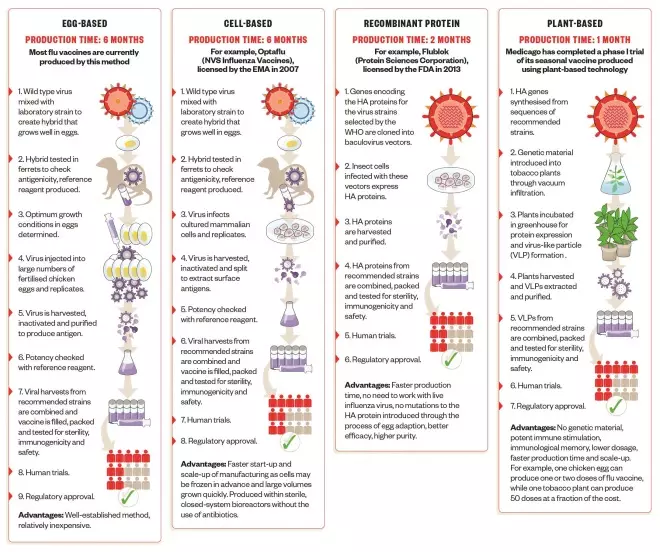
Speeding up production
Traditional egg-based vaccine production is more than 70 years old, takes around six months and uses millions of chicken eggs. Alternative production processes have been developed that aim to speed up manufacturing time and scale-up, as well as improve vaccine efficacy. Importantly, these processes do not rely on eggs, which could be in scarce supply if a bird flu pandemic hits.
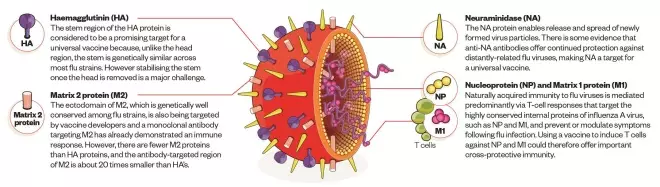
Working towards a universal vaccine
Many researchers are trying to create a vaccine that provides broad protection against existing and newly emergent strains, eliminating the need for annual vaccination. Some universal flu vaccines aim to stimulate a cellular immune response, as opposed to the antibody-based response targeted by current vaccines. A cellular response is the mechanism underlying naturally acquired immunity. Even a vaccine more broadly protective within a single subgroup would offer advantages, and could provide some intra-seasonal protection, most likely in combination with conventional vaccines.
References
Sources: Public Health England, EuroMOMO (Vaccine effectiveness), World Health Organization, NVS Influenza Vaccines, Protein Sciences Corporation, Medicago (Speeding up production), World Health Organization (Working towards a universal vaccine).
Infographic: AlisdairMacdonald.co.uk.
Editorial advisers: Sarah Gilbert, Rob Lambkin-Williams.