To see the full infographic, click here.
Safety features
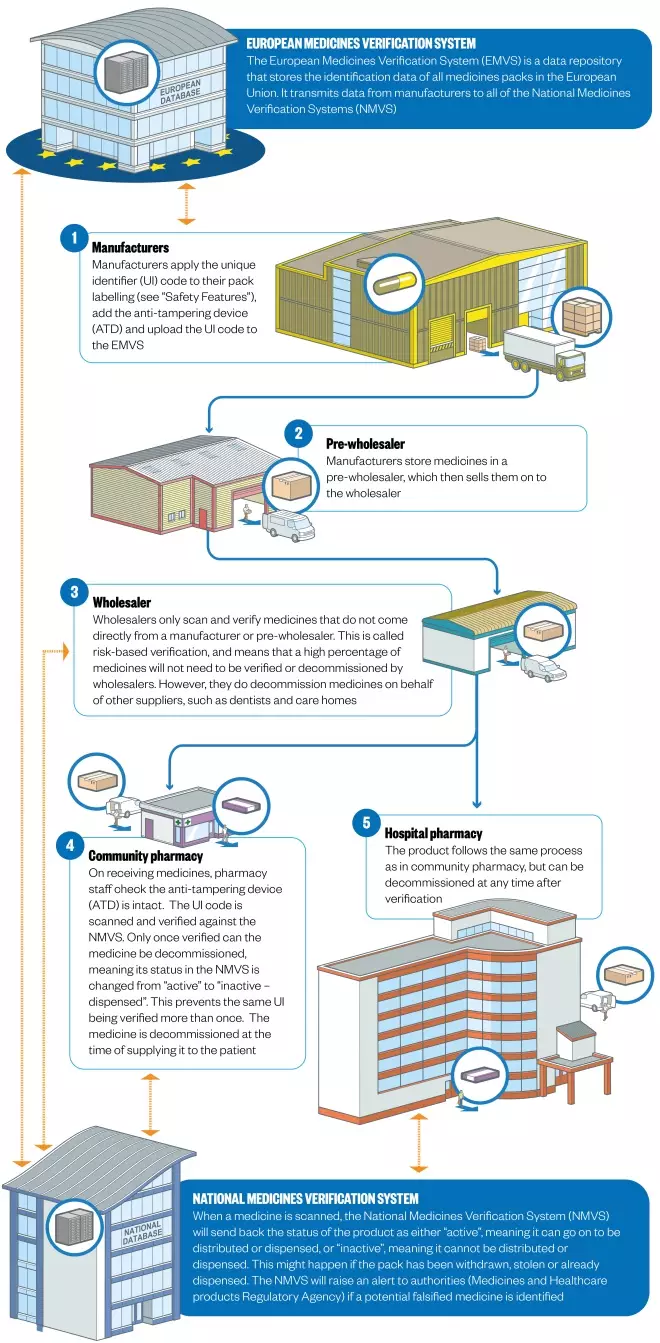
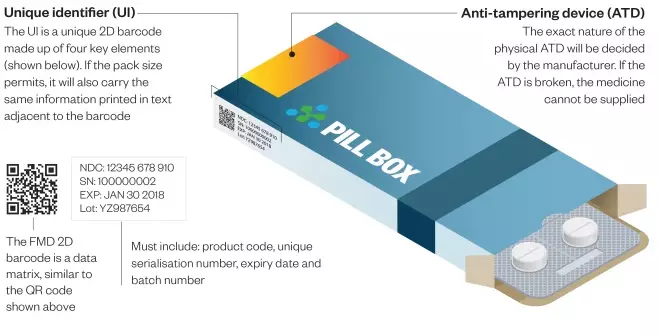
Under the FMD, all new packs of prescription medicines put on the market from February 2019 will have to have two safety features: a unique identifier (UI) and an anti-tampering device (ATD)
Who will be impacted by the FMD in the UK?
- 15,000 community pharmacies
- 260 acute NHS hospitals
- 190 private hospitals
- 1,200 dispensing doctors
- 7,500 GP practices and surgeries
- 1,500 authorised wholesalers/distributors
- 790 market authorisation holders
- 66 million patients
Falsified Medicines Directive
The Falsified Medicine’s Directive (FMD), which comes fully into force in February 2019, introduces EU-wide legislation to help prevent counterfeit prescription medicines entering the pharmaceutical supply chain. “Falsified” covers not only counterfeit medicines, but also false information about a medicine’s source. FMD will not cover non-prescribed medicines, with the single exception of omeprazole.
All medicines packs will be tamper-proof and will feature a 2-D barcode containing a unique serial number as well as a product code, batch number and expiry date.
Since 2011, the Royal Pharmaceutical Society and other stakeholders have been acting to ensure that the FMD will function in accordance with UK law, in a way that ensures patient safety, whilst minimising disruption to existing pharmacy practise.
The RPS has been acting on FMD since 2011, when it set up a multidisciplinary, cross-sectorial, GB-wide working group to develop policy. Later, in July 2014, the Society hosted and chaired the first FMD stakeholders networking meeting, at which 13 countries were represented by 100 delegates. At this meeting, implementation challenges for FMD were discussed, and presentations from the three main software providers were made.
Specific features of FMD in the UK lobbied for and achieved by the RPS and other stakeholders include:
- Extension of the originally-proposed two-day window for scanned products that are not collected by a patient to be returned to stock. This has been increased to ten days. Under FMD medicine not collected within the window will have to be destroyed, so extending the time limit reduces wastage.
- Agreement on the form of the European Stakeholder Model, which lays out a EU-wide format for how medicines can be verified, and on the European Medicines Verification System (EMVS), which guarantees authenticity from manufacturing to dispensing (the “end-to-end” system)
- Inclusion of human readable data alongside the 2-D barcode, so that pharmacists can continue to help patients if scanners break down or if the barcode is damaged.
- Full repackaging of imported medicines, rather than addition of new barcodes to existing packaging. Multiple barcodes could result in incorrect barcodes being scanned, triggering a false exceptional event.
- Greater flexibility offered to hospitals on the timing of medicines decommissioning. FMD requires medicines to be decommissioned at the point of dispensing. In hospitals – particularly in A&E, and where single doses are administered – this isn’t practical. Following lobbying, hospitals (unlike community pharmacies) will be able to decommission upon delivery, and then store medicines.
- Use of a single workstation. FMD originally required two, one for decommissioning and one for dispensing. RPS argued that this was unnecessary, costly, and increased the chance of technical failures disrupting processes. Successful lobbying led to agreement that all tasks can be performed on the Patient Medical Record (PMR) computer.
In addition, the RPS continues to call for the addition of safety information (including batch code, expiry dates and potential interactions) into the 2D barcode, alongside authentication data.
Your Society working for you.
Not a member? Become a member now
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
Infographic: Mark Watkinson
Editorial advisers: Jerome Bertin, UK general manager, SecurMed, and Martin Sawer, executive director, Healthcare Distribution Association (HDA UK)
Sources: Association of the British Pharmaceutical Industry, Healthcare Distribution Association, National Pharmacy Association, Pharmaceutical Services Negotiating Committee, Royal Pharmaceutical Society, SecurMed UK, TraceLink, UK FMD Working Group for Community Pharmacy, J Generic Med 2014;11:169