Download the full infographic here
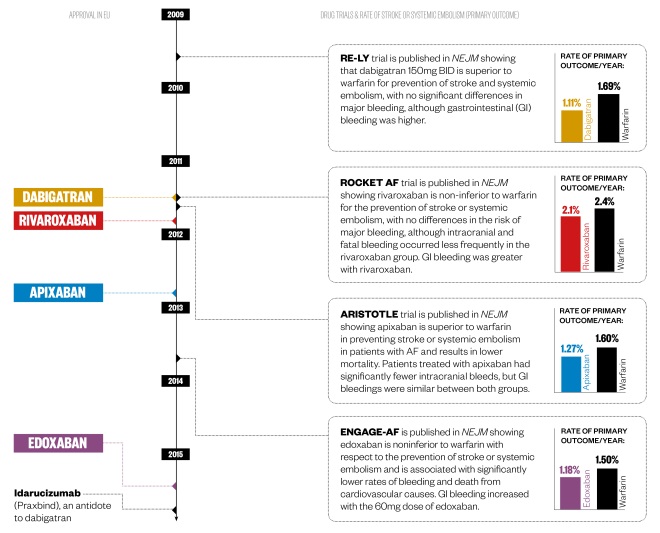
Timeline of key events
Dabigatran and rivaroxaban were approved in the EU in 2011 following publication of pivotal phase III trials in The New England Journal of Medicine. Apixaban followed the year after, with edoxaban being approved in 2015.
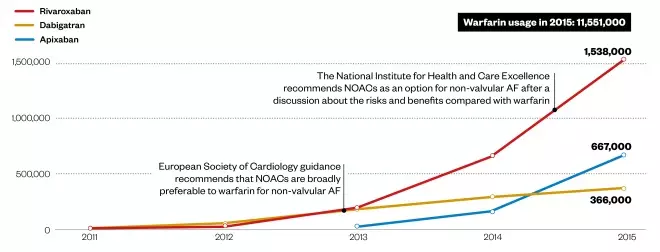
Slow rise of the new oral anticoagulants (NOACs)
Prescription items dispensed in the community in England. Edoxaban was approved in 2015.
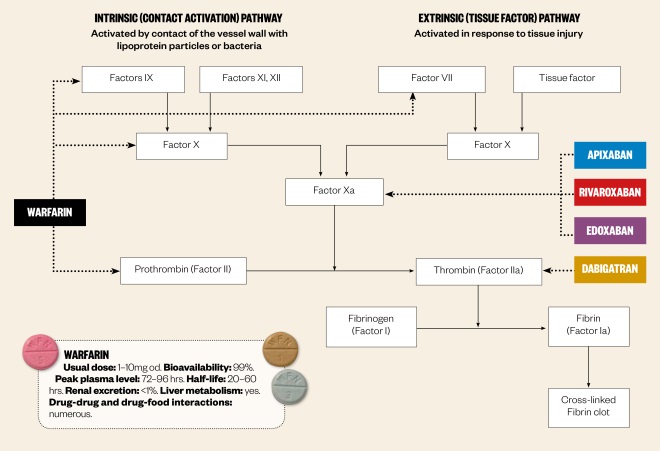
Anticoagulant effect of new oral anticoagulants (NOACs)
The coagulation cascade is a series of reactions involving coagulation factors that ultimately results in the formation of a blood clot. The NOACs directly inhibit one specific coagulation factor in the cascade, whereas warfarin prevents the coagulation process by suppressing the synthesis of several vitamin K-dependent coagulation factors.
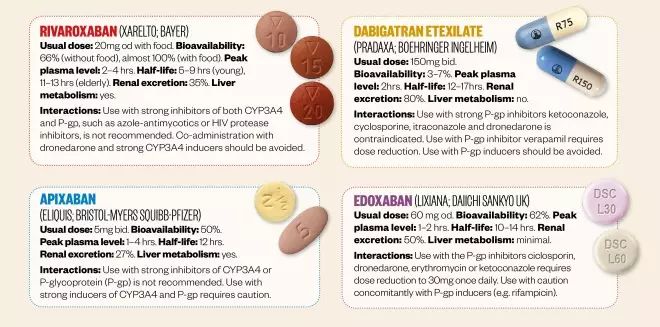
The new oral anticoagulants (NOACs)
All NOACs are indicated for the prevention of stroke and systemic embolism in adults with nonvalvular AF who have one or more risk factors, such as prior stroke or transient ischaemic attack; age ≥ 75 years; hypertension; diabetes mellitus and symptomatic heart failure. Unlike warfarin, the NOACs have a predictable therapeutic response, rapid onset of action, few drug interactions and no requirement for regular coagulation monitoring.

Deciding which new oral anticoagulant (NOAC)
Several patient characteristics may be considered when deciding on which NOAC to recommend. Patient preference for once daily dosing may also be taken into account. CAD: coronary artery disease, MI: myocardial infarction, ACS: acute coronary syndrome.
References
Sources: “Slow rise of NOACs” – NHS Digital; European Heart Journal; NICE; “Timeline” – New England Journal of Medicine; “The NOACs” – Europace; Summaries of product characteristics; “Deciding which NOAC” – Arrhythmia & Electrophysiology Review.
Editorial adviser: Satinder Bhandal, consultant anticoagulation pharmacist, Buckinghamshire Hospitals Trust
Infographic: MAG
You may also be interested in
Pharmacists made 150,000 patient interventions during anticoagulant safety audits, report finds
Some patients switched to edoxaban without being properly informed, pharmacists say