This content was published in 2012. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Summary
Around 20% of patients who are admitted to hospital acutely will develop acute kidney injury (AKI). The term describes a rapid deterioration in renal function and is characterised by reduced urine output and increased serum creatinine. The cause of AKI can be pre-renal (eg, hypovolaemia), intrinsic (eg, acute interstitial nephritis) or post-renal (eg, urinary obstruction).
AKI is largely preventable and pharmacists should take steps to optimise medicines for patients at high risk of the condition. This can involve providing advice about avoiding potentially nephrotoxic medicines, monitoring renal function and reviewing medicines.
It is believed that between 486 and 630 people per million of the population will develop acute kidney injury (AKI) in the UK each year.1 A fifth of people admitted acutely to hospital will develop AKI. Moreover, up to 30% of all cases of AKI are thought to be due to medicines, with some 5% of inpatients developing drug induced renal impairment.2
AKI has a high mortality rate — 10–80%, depending on complications that arise, the need for intensive care and renal replacement therapy (RRT). It has significant cost implications for the NHS: it is estimated that in 2009–10 between £434m and £620m was spent on managing the condition in England. In addition, inpatient stay was increased by an average of 4.7 days.3
In 2009, a report from the National Confidential Enquiry into Patient Outcome and Death (NCEPOD), entitled “Adding insult to injury”,4 sought to raise awareness of AKI. The report described many deficiencies in the care of patients who developed AKI and identified that only 50% of patients with AKI in UK hospitals received good care.
One deficiency highlighted was the inadequate assessment of risk factors for AKI — such as the use of potentially nephrotoxic medicines. In this article we will look at why AKI matters to us in practice and will consider the role of the pharmacy team.
Definition and staging
Formerly known as acute renal failure, AKI describes a rapid deterioration in a patient’s renal function over hours or days. It is defined as any of the following:
- Increase in serum creatinine of 26μmol/L within 48 hours
- Increase in serum creatinine greater than 1.5 times above a patient’s baseline that is known or presumed to have occurred within seven days
- Urine volume less than 0.5ml/kg/h for more than six consecutive hours
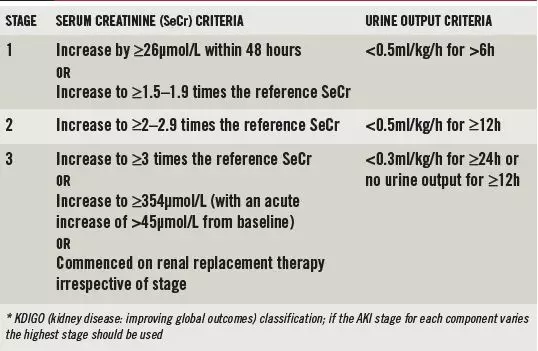
AKI is classified into three stages, depending on severity, using the KDIGO (kidney disease: improving global outcomes) criteria, outlined in Box 1. This international classification system is based on two former staging systems — RIFLE (risk, injury, failure, loss of kidney function and end-stage kidney disease) and AKIN (acute kidney injury network).
The definition of AKI and the staging system are based on the finding that even relatively small rises in serum creatinine are associated with poor outcomes,5 including prolonged hospital stay and increased morbidity and mortality.
Currently, a small number of hospitals in the UK have introduced AKI alerts linked to hospital biochemistry reporting systems. These systems compare patients’ serum creatinine values to previous results and alert clinicians to rapid deterioration in renal function based on the AKI staging classification.6 It is expected that such reporting systems will become more widespread.
Causes
Some 80% of AKI is caused by pre-renal issues and acute tubular necrosis;7 10% is due to obstruction (post-renal); and 10% is due to intrinsic renal causes.
Pre-renal causes
In pre-renal AKI blood flow to the kidney is reduced (as a result of gastrointestinal bleeding, sepsis, cardiac and liver failure, dehydration, burns, hypovolaemia, reduced blood pressure, etc). If pre-renal causes are not appropriately treated then ischaemic injury to the kidney can occur (namely, acute tubular necrosis).
Post-renal causes
Post-renal AKI is caused by obstruction to outflow from the kidneys (as a result of benign prostatic hypertrophy, prostate cancer, retroperitoneal fibrosis, renal calculi, etc).
Intrinsic causes
Intrinsic AKI is caused by damage to the functional tissues of the kidney and tends to be managed by specialist renal clinicians. Causes include:
- Acute interstitial nephritis — caused by a hypersensitivity reaction and often drug-induced; symptoms can include proteinuria, haematuria, eosinophilia, pyrexia, rash and arthralgia, but patients can present without these classic symptoms
- Myeloma
- Immunological renal disease, including vasculitis and glomerulonephritis
- Rhabdomyolysis — myoglobin from muscle breakdown is toxic to renal tubule cells; this is a rare cause of AKI
Box 1: Acute kidney injury stages*
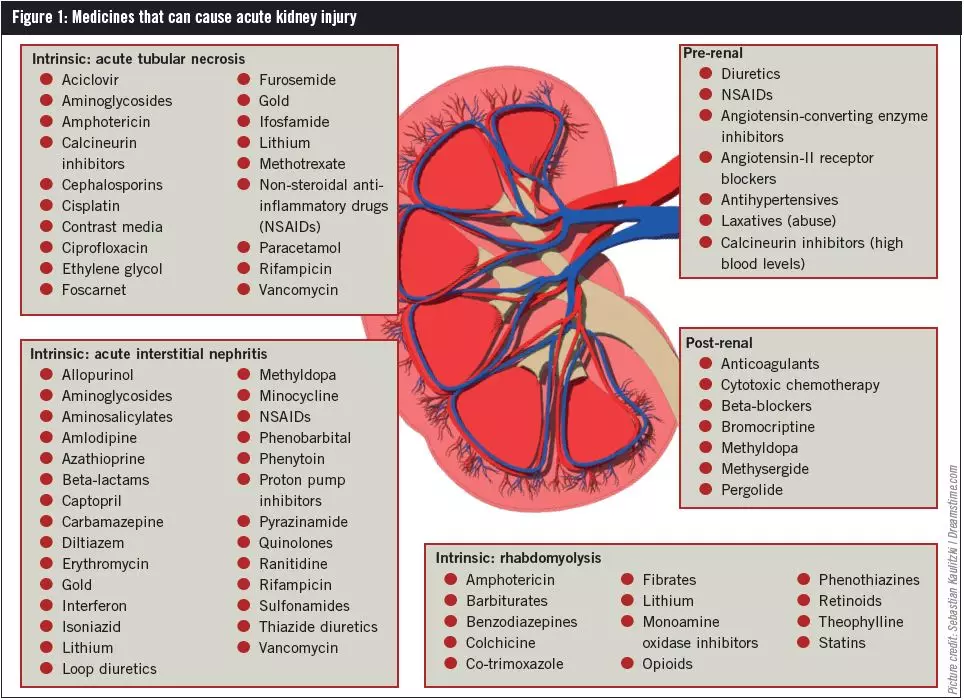
Drug-induced AKI
Many drugs can precipitate AKI. Acute tubular necrosis is the most common manifestation of drug-induced AKI and, although it can occur with normal drug doses, it more often results from high-dose treatment or accumulation of a drug due to pre-existing renal impairment. Drugs that have been known to cause acute tubular necrosis are set out in Figure 1.
Medicines particularly associated with renal hypoperfusion and pre-renal AKI include non-steroidal anti-inflammatory drugs (NSAIDs), angiotensin converting enzyme (ACE) inhibitors, angiotensin-II receptor blockers (ARBs) and diuretics. The body’s normal response to a reduction in renal blood flow is to restore glomerular filtration rate (GFR) through:
- Vasodilation of afferent blood vessels — release of prostaglandins dilates the vessels supplying blood to the kidneys
- Vasoconstriction of efferent blood vessels — activation of the renin angiotensin system constricts the blood vessels that leave the kidneys
However, NSAIDs inhibit prostaglandin synthesis and this results in afferent vasoconstriction. ACE inhibitors and ARBs cause vasodilation of efferent blood vessels, resulting in GFR lowering and AKI in susceptible patients, such as those with reduced perfusion pressure from hypotension or hypovolaemia and patients with renal artery stenosis. The use of diuretics can increase a patient’s risk of AKI by exacerbating hypovolaemia and reduced renal blood supply. Used alone, these medicines increase the risk of AKI; moreover there is a cumulative risk if they are used concomitantly.
Contrast-induced AKI, also termed contrast-induced nephropathy (CIN), can occur when patients are given iodine-based contrast media for radiological procedures, such as angiograms. In CIN, an acute increase in serum creatinine is seen one to two days following the procedure, peaking within three to four days. The dose and type of contrast given can affect the incidence of CIN; other contributing factors include pre-existing chronic kidney disease (CKD), low effective circulating blood volume and the use of other nephrotoxic medicines (see Box 2). Acute interstitial nephritis is often drug-induced (see Figure 1).
Mechanical blockage can cause drug-induced postrenal AKI. For example, anticoagulants can cause bleeding leading to blood clot formation and ureteric obstruction. Use of cytotoxic drugs can result in deposition of urate crystals in the renal tubules to such an extent that blockage occurs. Other medicines that can cause postrenal AKI are listed in Figure 1.
There are many factors involved in drug-induced AKI, with some drugs having more than one mechanism for causing damage (eg, NSAIDs can cause vasoconstriction, interstitial nephritis and proteinuria).
Diagnosis
For patients with AKI, rapid reduction in kidney function results in a failure to maintain fluid, electrolyte and acid base homeostasis. If these are not corrected quickly patients may go on to develop uraemic complications, pulmonary oedema, hyperkalaemia and metabolic acidosis.
AKI is typically diagnosed based on a fall in urine output (oliguria or anuria) or a rise in blood serum creatinine. The NCEPOD for AKI recommends that all patients admitted acutely to hospital should have their urea and electrolytes measured and assessed.4 If a patient is found to have AKI, the cause must be identified. Early diagnosis is essential so that the underlying pathology can be treated and the need for renal replacement therapy mitigated.
The diagnostic process starts with a detailed history, including a drug history. All patients with AKI should undergo urinalysis to differentiate between pre-renal AKI and the different forms of intrinsic AKI. If a urine dipstick is positive for protein and nitrites, and the patient has symptoms to suggest a urinary tract infection, a midstream urine sample should be taken and then appropriate antibiotics started. If urinalysis is positive for protein, the urinary albumin-to-creatinine ratio should be measured to establish the amount of protein in the urine.
A patient’s vital signs should be assessed, including temperature, blood pressure (including postural changes in blood pressure) pulse and urine output. A full blood count should be taken, including white cell count to monitor for signs of sepsis. Hydration status should be assessed carefully, paying attention to signs and symptoms of both dehydration and fluid overload. Signs of hypovolaemia include a reduced jugular venous pressure (JVP) or central venous pressure (CVP), increased heart rate and reduced blood pressure. Signs of hypervolaemia include a raised JVP or CVP, increased heart rate, increased or decreased blood pressure, reduced oxygen saturation and increased respiratory rate. It may be necessary to perform a chest X-ray to check for pulmonary oedema. (More information about fluid balance can be found in Clinical Pharmacist 2011;3:274.)
Ultrasound of the bladder is performed if urinary obstruction is suspected, or in more severe AKI (stages 2 and 3) or if AKI is not improving.
It should be noted that AKI that occurs secondary to systemic disease can present with additional clinical features such as fever, rash and joint pain.
Specialist nephrology input
Referral to a nephrologist should be considered for patients:
- With stage 3 AKI
- With indications for renal replacement therapy
- In whom an intrinsic cause of AKI is suspected
- Who are not improving despite actions to correct pre-renal causes of AKI
Prevention
AKI is often avoidable. It is estimated that 20% of AKI
that occurs after patients are admitted to hospital is
predictable and avoidable.3 Risk factors for AKI include:
- Age — AKI can occur at any age, but it is more common in patients over 75 years of age
- Long-term disease — particularly pre-existing CKD (eGFR <60ml/min/1.73m2), but also diabetes mellitus, congestive heart failure, liver disease and atherosclerotic peripheral vascular disease
- Surgical procedures — risk is higher for those undergoing cardiac surgery and patients in whom fluid balance is not appropriately assessed before, during and after the procedure
- Medication (see Box 2)
NHS Kidney Care focuses on the three Rs —reducing risk, early recognition and right response — to lower the incidence of AKI and the need for referral for specialist renal care. Preventing AKI and reducing risk involves:
- Early assessment
- Treating infections and other medical conditions promptly
- Ensuring patients are hydrated appropriately
- Avoiding hypotension (systolic BP <110mmHg)
- Medicines optimisation — the NCEPOD AKI report4 noted that not all patients with AKI had nephrotoxic drugs stopped and drug doses were not commonly altered (see Box 2)
Prognosis
Mortality rates increase sharply as AKI worsens, and can be as high as 50% for patients with multi-organ failure. For many patients who survive, their renal function does not return to that seen before AKI; persisting renal dysfunction reduces long-term survival. Patients who develop CKD, with or without ongoing need for RRT, should be managed according to local and national guidelines (see Clinical Pharmacist 2011;3:15).
Box 2: Medicines optimisation and AKI
There are three key steps in reducing the risk of AKI through medicines optimisation:
Step 1 — avoid nephrotoxic medicines If possible, nephrotoxic medicines should not be used for patients with chronic kidney disease (CKD). For example, non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided in CKD and pharmacists should offer advice about over-the-counter and alternative analgesics for such patients (considering their side effect profile and renal excretion).
Step 2 — monitor renal function Clinicians should be alert for deterioration in renal function when medicines with the potential to be nephrotoxic are started or their dose is increased. Such medicines include angiotensin-converting enzyme (ACE) inhibitors, angiotensin-II receptor blockers (ARBs), diuretics (for spironolactone also monitor for hyperkalaemia) and aminoglycosides (ensure therapeutic drug monitoring is performed for all patients).
Step 3 — review nephrotoxic medicines The need for nephrotoxic medicines should be reviewed in patients at risk of AKI. If patients become acutely unwell (eg, through dehydration due to diarrhoea and vomiting) they are at an increased risk of AKI. Ensure patients are adequately hydrated and that their medicines are reviewed regularly. It may be appropriate to withhold medicines such as ACE inhibitors, ARBs, diuretics and NSAIDs until the acute illness resolves (eg, for 24–48 hours).
Patients undergoing radiological procedures that require contrast media, eg, angiography, may also be at risk of AKI. Patients at risk should receive low osmolar contrast agents, the choice of which will likely be determined by the radiologist. Adequate hydration with sodium chloride (0.9% or 0.45%) or sodium bicarbonate is essential before and following the use of contrast media. Although evidence for the use of acetylcysteine for the prevention of contrast-induced nephropathy is limited, patients with CKD who are at risk of AKI can be given 600mg orally twice daily on the day before and the day of the procedure.
Before any procedure, patients should be given information about medicines that they should temporarily stop taking, such as ACE inhibitors, ARBs, diuretics and NSAIDs. Some units will recommend that metformin is temporarily withheld because of the risk of lactic acidosis if AKI develops.
References
- Lewington A, Kanagasundaram S. UK Renal Association clinical practice guideline: acute kidney injury. March 2011. www.renal.org/Clinical/ GuidelinesSection/AcuteKidneyInjury.aspx (accessed 1 February 2012).
- Ashley C, Morlidge C. Introduction to Renal Therapeutics. London: Pharmaceutical Press; 2008.
- Taylor J (ed). Acute kidney injury. Health Service Journal supplement; 23 June 2011.
- National Confidential Enquiry into Patient Outcome and Death. Adding insult to injury: a review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure). 2009 www.ncepod.org.uk/2009aki.htm (accessed 1 February 2012).
- Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology 2005;16:3365–70.
- Selby NM, Crowley L, Fluck RJ, et al. Use of electronic results reporting to diagnose and monitor acute kidney injury in hospitalized patients. Clinical Journal of the American Society of Nephrology (online February 2012).
- Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney International 1996;50:811–8.