This content was published in 2003. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
How can we meet the ever increasing demand for health care, given our limited resources? Health economics helps to address this issue. It can be seen as a tool to help prioritise health care interventions so that maximum health benefits are provided to the population within the resource constraints that exist. The key concept underlying health economics is that an effective health care intervention, such as the prescribing of an appropriate drug, can be recommended for patients who will benefit, but if the drug is not cost-effective these patients will be using resources that would produce greater benefit for other patients at the same cost.
In the face of rising expenditure, the National Health Service has sought to develop ways to prioritise the available health care resources, so as to provide the most health benefits to the population that it serves. In 1999, the creation of the National Institute for Clinical Excellence (NICE) signalled this move towards clinical practice decisions based on evidence of clinical effectiveness as well as cost-effectiveness. Economic evaluations are tools that health economists use to assess the cost-effectiveness of health care interventions. This involves looking at both the benefits and the costs of interventions. With most new health technologies or a new drug, extra clinical effectiveness will be associated with an extra cost. Once deployed, the resources used to meet these extra costs are no longer available for other interventions.
Economic evaluations help decision makers determine whether the cost of this extra effectiveness provided by the new drug is worthwhile, within the budget available. They are not supposed to replace the judgement of health professionals, but instead should support both health professionals and decision makers in making informed decisions.
Costs in economic evaluations
Evaluating the costs of a health care intervention first requires the perspective of the economic evaluation to be defined. This will determine which categories of cost are relevant to the analysis. A number of perspectives can be adopted in an economic evaluation. For example, an evaluation could be conducted from the viewpoint of the provider institution, the purchaser of health care, the health service or society. As an illustration, an analysis from the perspective of a hospital might include staff costs, hospital stay costs (also referred to as “hotel costs,” these include costs of cleaning, heating, laundry and meals), drug costs and diagnostic testing costs. In comparison, taking a wider NHS perspective would mean including other costs, such as prescription costs for drugs used by the patient after discharge. Health economists agree that economic evaluations should be conducted from the perspective of society, which includes all costs associated in the evaluation, for example, the cost to the patient of time off work. However, in practice, evaluations tend to be conducted from more narrow points of view.
The following terms are ones that may be encountered in the description of the categories of cost in economic evaluations.
Direct costs
Direct costs are those directly associated with the health care intervention. These can be divided into (i) direct health care costs, such as nurse and doctor salaries, drug costs, the cost of laboratory tests needed for the intervention; and (ii) direct non health care costs, such as the patient’s cost of transportation to and from treatment centres.
Indirect costs
Indirect costs are associated with reduced productivity due to illness, disability and death. They are typically calculated from the gross earnings of those in employment. If the analysis is conducted from society’s perspective, indirect costs should be included but, in practice, these costs are often ignored.
Intangible costs
Intangible costs relate to psychological costs associated with illness or treatment, such as pain and suffering. Although these costs may be mentioned in economic evaluations, they are rarely quantified because of the practical difficulties involved in doing so.
Once the perspective of a study has been defined, the amount of resources the intervention uses need to be measured. Examples of measures include staff time needed to administer the treatment, the quantity of drugs consumed or the number of days in hospital. In economic evaluations conducted alongside clinical trials, this information can be collected directly. Other methods estimating the quantity of resources used involve collecting data prospectively or retrospectively from medical records. The costing is completed by applying prices (“unit costs”) to the quantities of resources measured. These figures can be obtained from the finance departments of particular institutions or from national statistics.
In relation to costs in economic evaluations, readers may come across various other terms and these are explained below.
Opportunity cost
Opportunity cost is a central notion in economic analysis. It can be used explain the consequences of choosing between two alternatives. Imagine we have a choice of two effective treatments, A and B, but only enough money for one of them. If treatment A is funded rather than treatment B, the opportunity cost of funding A is the benefits we forgo in not choosing B, the next best alternative use of the resources. Consider, for example, two possible interventions: a cancer screening programme (intervention A) and the next best alternative, a smoking cessation programme (intervention B). Only one of these interventions can be funded within the available budget. The opportunity cost of funding A can be thought of as the number of life years that would have been gained through the smoking cessation programme. Opportunity costs reflect the fact that choices have to be made between interventions because of the scarcity of resources.
Average cost
Average cost is calculated by dividing the total costs for the intervention by the total quantity of treatment units provided, such as the number of patients receiving a course of antibiotics.
Incremental cost
Incremental cost is the difference in costs found between two interventions (ie, the intervention and its comparator).
Marginal cost
Marginal cost is the extra cost of producing one unit of output or expanding a programme or service (eg, increasing the length of stay in hospital by one day).
Types of economic evaluations
There are four main types of economic evaluation: cost-minimisation analysis, cost-effectiveness analysis, cost-utility analysis and cost-benefit analysis. Although they employ similar methods to define and evaluate costs, they differ in the methods used to estimate the benefits from a programme or intervention. This section looks at each in turn.
Cost-minimisation analysis
Cost-minimisation analysis (CMA) compares the costs of different interventions that are assumed to provide equivalent benefits. A good example would be a comparison between a generic drug and its branded equivalent. If the assumption of equal effectiveness is substantiated, the decision hinges on finding the least expensive way of obtaining that health benefit — only the costs are compared and not the benefits. The decision rule is therefore simple because the cheapest intervention will provide the best value for money. However, in practice, there are relatively few CMAs because it is rare for two health care interventions to provide exactly the same benefits.
Cost-effectiveness analysis
In cost-effectiveness analysis (CEA), benefits are measured in natural units. For example, these could be heart attacks avoided, deaths avoided or life-years gained (ie, the number of years by which life is extended as a result of the intervention). Quality of life scores are also used. These can be obtained from health related quality of life (HRQoL) instruments that measure the quality of life of the patient in a number of domains (eg, physical, emotional and social) and provide scores for each. The main restriction in CEA is that it is one dimensional — only one domain of benefits can be explored at a time. This can pose a problem because it can be difficult to choose which single outcome best represents the intervention. One possibility is to conduct a cost-consequence analysis. This is a particular type of CEA that evaluates multiple outcomes and reports costs and benefits in a disaggregated form, leaving the reader to decide on which benefit to select.
Cost-utility analysis
In cost-utility analysis (CUA), the benefits are measured in healthy years, to which a value has been attached. Unlike CEA, CUA is multidimensional and incorporates considerations of quality of life as well as quantity of life using a common unit. For example, in studies looking at hormone replacement therapy, benefits such as reduction in fracture risk and alleviation of menopausal symptoms can be incorporated alongside risks such as stroke or breast cancer.
Valuing healthy years reflects that a preference has been expressed for being in one health state rather than another. In health economics, a “utility” is the measure of the preference or value that an individual or society places upon a particular health state. It is generally a number between zero (representing death) and one (perfect health). Utilities can be measured using direct methods such as the “standard gamble” or “time trade off”.
The standard gamble is a technique widely used in economics. It is based on the idea that something is only of value if we are prepared to give another thing up in order to get it. The respondent is asked to make a trade-off between the certainty of having a chronic disease for a period (t) and a gamble that has two possible alternatives: staying in good health for the same period or death. Finding the point where the respondent is indifferent between being in the chronic condition and the gamble provides us with a value that reflects the quality of life that the respondent attaches to the chronic condition.
Time trade off techniques are based on concepts similar to the standard gamble. The respondent is asked how many years of life in a health state with a disease, he or she would be willing to give up to be in full health but for a shorter period. For example, the respondent may be asked if he or she would prefer to live five years in health state with a specified chronic condition, or three years in perfect health. The process goes on until the point at which the respondent is indifferent between the two health states is found.
Because these techniques are complex, simpler methods have been devised to obtain health state utilities. Generic health state questionnaires (eg, Euroqol) ask respondents a number of simple, health-related questions and then convert the results into utilities using pre-scaled responses obtained by standard gamble or time trade off, from a relevant reference group. Health state utilities can be elicited directly from patients, but when this is not possible significant family members, caretakers or health professionals may be also be asked to respond.
The most widely used measure of benefit in CUA is the quality adjusted life year (QALY), but other measures include disability adjusted life years (DALYs) and healthy year equivalents (HYEs). These are described in Panel 1.
The results of cost-effectiveness and cost-utility analyses can be expressed in several ways. If benefits are shown to be equivalent, then the analysis is really a CMA and the intervention with the lowest cost should be chosen. If one intervention is both cheaper and more effective than its comparator, the intervention is said to be dominant and should be chosen, since it will provide larger benefits at a lower cost than its comparator. However, most commonly, an intervention will be found to be more effective but will also be more costly than the comparator. In order to make a decision on which treatment to select, the incremental cost-effectiveness ratio (ICER) should be calculated. This is done as follows:
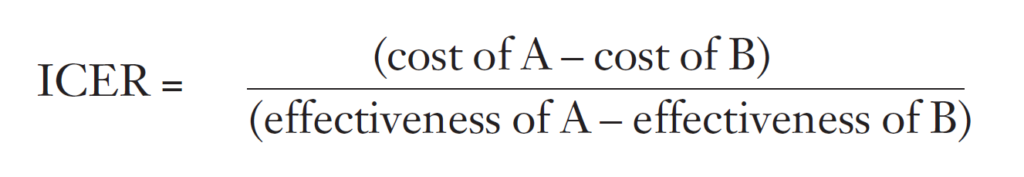
Panel 1: Measures of benefits
Quality adjusted life years
A quality adjusted life year (QALY) is a common measure of benefit that combines quantity and quality of life. It is calculated by estimating the total number of life-years gained from treatment and weighting each year with a quality of life score (or utility) to reflect the quality of life in that year. For example, a patient living for 10 years but with a quality of life of say, 0.7 on a scale of 0 to 1 (with 0 as death and 1 as perfect health), would live for seven (0.7 x 10) QALYs (this simplified example assumes no improvement or deterioration in the quality of life of the patient during these 10 years). How the utility score has been obtained is described in the text above.
Disability adjusted life years
A disability adjusted life year (DALY) is another indicator, similar to QALYs, that was developed by the World Bank and the World Health Organization to quantify the global burden of disease. Like a QALY, it incorporates both quantity and quality of life in a common measure. The main difference is that it measures losses of healthy life rather than life-years gained. However, DALYs have been subject to a number of criticisms as a measure of health status and as a tool for setting health priorities and are not encountered often in economic evaluations published in the United Kingdom.
Healthy year equivalents
Healthy year equivalents (HYEs) also provide a measure of quantity and quality of life. Whereas QALYs weigh each year lived in a health state independently, HYEs consider a sequence of health states and their duration and then ask respondents how many healthy years of life this scenario is equivalent to. For example, a respondent could be asked the following: if you live with a disabling hip fracture for three years, how many years of healthy life would this be equivalent to? In contrast a QALY approach would ask the respondent to rate one year lived with a disabling hip fracture, irrespective of whether he or she had the condition for one year or 10 years. In practice, HYEs have not been used often because of the complexities involved in their measurement.
This ratio tells us the cost per extra unit of effectiveness of A over B. The next question to ask is if this more effective programme is affordable. The difficulty with economic evaluations is that there is no magic cut-off number that establishes whether or not an intervention is cost-effective. In fact, the cost-effectiveness of an intervention will depend on what is termed the decision maker’s “ceiling ratio”. This ceiling ratio can be inferred from the amount that decisionmakers in the NHS are willing to pay for health interventions. For example, if the incremental cost of treatment A is £10,000 per QALY compared with treatment B and the decision-maker recommends it, then we can infer that the ceiling ratio is at least £10,000 per QALY. Although we do not have an exact figure for this ceiling ratio, a study of the decisions made by NICE showed that interventions seemed to be recommended for values at or below £30,000 per QALY[1].
Cost-benefit analysis
In cost-benefit analysis (CBA), the benefits are measured in monetary terms. The final result is expressed as a net monetary gain (or loss) or as a cost:benefit ratio. There are two main methods to measure health gains for CBA: the human capital approach and the willingness to pay approach.
The human capital approach values a health improvement on the basis of future productive worth to society from being able to return to work. Values have to be added for activities that are outside traditional definitions of paid work, such as staying at home, being retired or unemployed, so this approach suffers from problems of how to value a number of health improvements. This is a narrow view of the value of improved health and is now not often used.
The willingness to pay approach seeks to establish the value that people attach to health care outcomes by asking them how much they would be willing to pay to obtain the benefits or avoid the costs of illness. A number of problems remain with this approach, such as the link between willingness to pay and ability to pay, so that the applications of the approach are relatively rare.
In CBAs, the decision rule on whether to fund an intervention is simple: if the benefits of implementing the programme are greater than the costs, then the programme should be funded. Theoretically, CBAs can provide information on whether a health programme is worthwhile funding from the point of view of society, in comparison to other health programmes, but also in comparison with other areas of social policy such as the environment and transport. However in practice, CBAs are rarely used in health care because of the difficulties of expressing health benefits directly in monetary terms.
Other types of economic evaluation
Burden of disease and cost-ofillness studies quantify the burden of a disease in monetary terms. For example, for osteoporosis, the number of fractures occurring and their total cost to the NHS would be evaluated. However, what is relevant is the relative costs and benefits of different treatments for the condition, rather than the overall cost of the disease to society. Because these types of studies only look at the costs associated with a condition and not at the benefits, they are not true economic evaluations. Indeed, allocating resources to the diseases with the highest burden will not necessarily result in an efficient use of resources, if the interventions available for that disease or condition are not cost-effective. The next article looks at how to assess an economic evaluation and the policy uses of pharmacoeconomics.
References
- Raftery J. NICE: faster access to modern treatments? Analysis of guidance on health technologies. BMJ 2001;323:1300–3.