Dr P Marazzi / Science Photo Library
Antiphospholipid syndrome (APS, also known as Hughes syndrome) is an autoimmune condition in which patients are in a hypercoagulable state, which means that they are at increased risk of developing thromboses in the venous, arterial or microvascular system[1]
. APS is the most commonly acquired thrombophilia and contributes to the development of several acute conditions. The exact prevalence of APS is currently unknown, however, the Antiphospholipid Syndrome Alliance For Clinical Trials and International Networking (APS ACTION) group have estimated the prevalence of antiphospholipid antibodies (aPL) and specific clinical presentations to be:
- 13.5% of patients with stroke;
- 11.0% of patients with myocardial infarctions (MI);
- 9.5% of patients with deep vein thrombosis (DVT) and;
- 6.0% of patients with pregnancy morbidity
[2]
.
APS is classified into two forms: secondary APS occurs in the presence of related autoimmune conditions, such as systemic lupus erythematous (SLE) or rheumatoid arthritis, whereas primary APS occurs in the absence of these conditions[3]
.
Pregnancy complications are a hallmark of APS, with successful pregnancy, resulting in a live birth, reported to be as low as 10% in women who have had no pharmacological intervention[4]
.
Pathogenesis, pathophysiology and risk factors
Several cellular pathways and events are associated with APS pathogenesis. For a detailed summary of the proposed mechanism of aPL-mediated thrombosis, see Figure 1. A group of aPL that bind to phospholipid binding proteins (most commonly β-2 glycoprotein 1, but also prothrombin, tissue plasminogen activator, annexin A2 and thrombin[5]
) may be associated with the activation of endothelial cells, monocytes and platelets. This activation results in the following mechanisms:
- Endothelial cells increase the expression of adhesion molecules (e.g. intracellular cell adhesion molecule-1, vascular cell adhesion molecule-1, E-selectin and upregulation of production of tissue factor)[6]
; - Monocytes upregulate the production of tissue factors[6]
; - Platelets increase the expression of glycoprotein IIb-IIIa receptors and the synthesis of thromboxane A2[6]
.
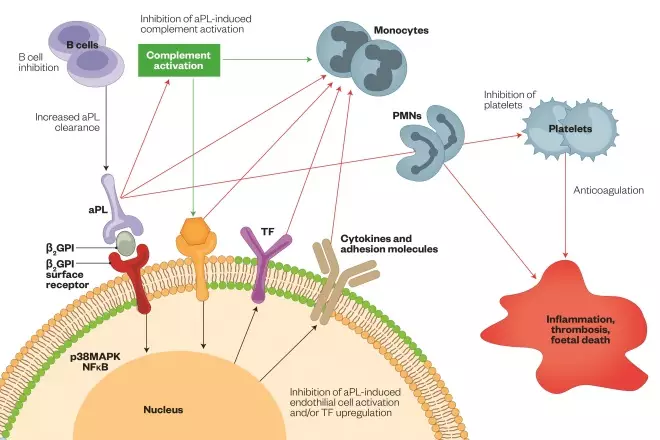
Figure 1: Proposed mechanism aPL-mediated thrombosis
Reproduced with permission from: Erkan D & Lockshin MD. New approaches for managing antiphospholipid syndrome. Nature Clinical Practice Rheumatology 2009;5:160–170. doi: 10.1038/ncprheum1017
Cell membranes comprise neutral phosphatidylcholine phospholipids (green circles), which are the major constituent of the outer layer of inactive cells, and negatively charged phosphatidylserine phospholipids (orange circles), which migrate from the inner to the outer cell membrane during activation or apoptosis of platelets and endothelial cells. Dimeric β2GPI then binds to phosphatidylserine phospholipids (via β2GPI surface receptors, such as apoER2’, annexin A2 or Toll-like receptors) and aPL bind to β2GPI, activating the complement system and leading to the generation of C5a, which induces expression of adhesion molecules (e.g. ICAM1), cytokines (e.g. IL-1, IL-6, IL-8) and TF, and activation of monocytes, PMN cells and platelets, which subsequently results in the release of proinflammatory mediators (such as TNF or VEGFR1) and the prothrombotic state. Both NFκB and p38MAPK could have a role in the thrombotic intracellular signaling cascade. Numbers 1–6 represent the stages at which aPL can be inhibited. Abbreviations: aPL, antiphospholipid antibodies; apoER2’, apolipoprotein E receptor 2’; β2GPI, β2-glycoprotein-I; ICAM1, intracellular adhesion molecule 1; IL, interleukin; NFκB, nuclear factor κB; PMN, polymorphonuclear; p38MAPK, p38 mitogen-activated protein kinase; TF, tissue factor; TNF, tumour necrosis factor; VEGFR1, vascular endothelial growth factor receptor 1
These three pathways induce a thrombogenic state by increasing the synthesis of tissue factor and thromboxane A2[3]
,
which may lead to activation of the coagulation system and subsequent development of thrombosis[3]
. However, the mechanisms causing thrombosis are not currently fully understood. Other suggested mechanisms for its development include interference in the protein C anticoagulant pathway, inhibition of fibrinolysis and inhibition of annex V binding to phospholipids[3]
. For a summary of the pathogenesis of thrombosis in APS, see Figure 2.
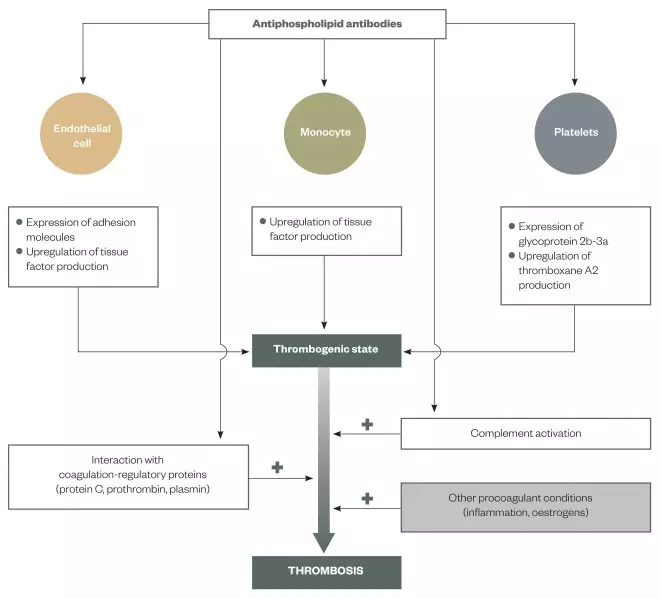
Figure 2: Pathogenesis of thrombosis in antiphospholipid syndrome (APS)
Reproduced with permission from: Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. The Lancet 2010;(376):1498–1509
Schematic of how the activation of endothelial cells, monocytes and platelets can induce the thrombogenic state and, subsequently, the development of thrombosis. As the mechanisms that cause thrombosis are not fully understood, other potential mechanisms (e.g. interference in the protein C anticoagulant pathway) are also depicted
Around 40% of patients with SLE have circulating aPL, however, fewer than half of these patients will develop thrombotic events[6]
. Younger patients presenting with clinical features of APS have a higher probability of carrying aPL compared with patients aged over 70 years who may have several additional vascular risk factors (e.g. stroke). It is currently unknown why some individuals carrying aPL present with thrombosis while others remain asymptomatic[5]
.
Complications in pregnancy can develop as a result of APS. This is commonly through the inhibition of trophoblastic function (the outer layer of cells that provide nutrients to the embryo), which can lead to miscarriage[4]
. Although other mechanisms may include a local inflammatory response (as a result of activation of the complement system) or, in later stages of pregnancy, the formation of thrombosis in the placental bed[4]
. However, it should be noted that other contributing factors can increase the risk of foetal death, (e.g. underlying hypertension, SLE or renal disease)[6]
.
Diagnosis
To be diagnosed with APS, will generally present with at least one clinical manifestation in addition to one positive laboratory test result. Box 1 highlights specific classification criteria for each category.
Box 1: Revised classification criteria for the antiphospholipid syndrome[1]
Clinical criteria
Vascular thrombosis
- One or more clinical episodes of arterial, venous or small vessel thrombosis, in any tissue or organ.
Pregnancy morbidity
- One or more unexplained deaths of a morphologically healthy foetus at or beyond the tenth week of gestation, with healthy foetal morphology documented by ultrasound or by direct examination of the foetus.
- One or more premature births of a morphologically healthy newborn baby before the 34th week of gestation because of eclampsia, severe preeclampsia or recognised features of placental insufficiency.
- Three or more unexplained consecutive spontaneous abortions before the tenth week of gestation, excluding maternal anatomical or hormonal abnormalities, and paternal and maternal chromosomal causes.
Laboratory criteria
- Lupus antiocoagulant present in plasma, detected according to the guidelines of the International Society on Thrombosis and Hemostasis[7]
,[8]
. - Anticardiolipin antibody of IgG or IgM isotype, or both, in serum or plasma, present in medium or high titres (i.e. >40 GPL or MPL, or greater than the 99th percentile), measured by a standardised ELISA.
- Anti-β2 -gycoprotein 1 antibody of IgG or IgM isotype, or both, in serum or plasma (in titres greater than the 99th percentile), measured by a standardised enzyme linked immunosorbent assay (ELISA).
- Detection of these antibodies on two or more occasions, at least 12 weeks apart.
Clinical criteria
Patients with APS typically present with DVT, pulmonary embolism (PE), stroke or MI[3]
. Patients can also present with other symptoms, such as migraine, memory problems, dizziness, visual disturbances and blotchy skin (livedo reticularis)[5]
.
Thrombosis of deep veins of the lower limbs and PE are the most common presentation of venous thrombosis. However, thrombosis of veins in other vascular beds (e.g. intra-abdominal superficial, portal, renal and intracranial) may also occur[3]
. Arterial thrombosis can manifest as strokes and, less commonly, MIs[3]
.
Pregnancy complications can include recurrent spontaneous miscarriages, intrauterine growth restriction, pre-term delivery and pre-eclampsia[6]
. It is recommended that patients who suffer from recurrent miscarriages in the first trimester, or one or more in the second trimester, be screened for aPL[4]
.
Laboratory criteria
For laboratory diagnosis, presence of aPL (specifically lupus anticoagulant [LA], anticardiolipin [aCL] and/or anti-β2-gycoprotein 1 antibodies) must be detected[3]
. LAs are recognised by the demonstration of prolongation of phospholipid-dependent inhibition of specific coagulation tests, such as the dilute Russell viper venom time[3]
. No single test is 100% sensitive to LA, therefore, repeat testing is recommended[1]
. Enzyme linked immunosorbent assays (ELISAs) are used to detect aCL and β2-gycoprotein 1 antibodies. If a positive result is obtained, the tests should be repeated at least 12 weeks later to ensure persistent positivity, however, this interval has not been validated[1]
.
Diagnosis of APS cannot be confirmed if the clinical event occurs ≥5 years from the positive aPL result[1]
. Circulating LA has been demonstrated to be the predominant antibody associated with thrombosis and pregnancy morbidity[3]
. Presence of LA can increase the risk of stroke by 40-fold and the risk of MI 5-fold[6]
. Anti-β2-gylcoprotein 1 has been associated with a 2-fold risk of stroke[6]
. However, triple positivity of these antibodies has been associated with the highest risk of clinical features[6]
; around 30% of these patients may present with recurrent events while on treatment[6]
.
Since there are limitations with aPL testing (e.g. reproducibility and standardisation), it is important that a thorough clinical history is taken at the point of diagnosis. There is broad variation in laboratory testing for aPL and positive readings of these antibodies can occur in the presence of infection, and poor sample collection or quality.
Other criteria
Other features shown to be associated with APS that are separate to the diagnostic criteria are:
- Heart valve disease (specifically mitral valve and aortic valve)[1]
; - Thrombocytopenia[1]
; - Nephropathy[1]
; - Neurological conditions and presence of other antibodies[1]
.
Cognitive impairment has been associated with the presence of aCL[1]
. Coronary artery disease can develop as a result premature atherosclerosis associated with aPL, however, the development of heart valve disease associated with APS is unknown[8]
.
Catastrophic APS
Thrombosis within the microvasculature is not as common as the other clinical features described above, because it presents in fewer than 1% of APS patients[5]
. When this occurs in multiple organs within a short time interval, it can be life threatening and is termed ‘catastrophic APS’ (CAPS)[3]
. CAPS is associated with a greater than 50% mortality[6]
.
Treatment
Antithrombotic therapy is the cornerstone of APS treatment; drug choice is dependent on the clinical event and risk stratification[5]
. Lack of randomised controlled trials in this small proportion of patients has led to treatment options being largely based on observational studies[9]
[10]
.
Where anticoagulation is indicated, there is debate on the intensity and duration of warfarin therapy and individual patient parameters should be taken into consideration[4]
. Table 1 provides a summary of treatment options for different groups of patients. Baseline bloods (e.g. INR, LFTs and APTT) need to be taken to rule out other coagulation disorders.
| Patient population | Treatment regimen |
|---|---|
| Asymptomatic antiphospholipid antibodies (aPL) positive | Low risk: Lifestyle changes High risk: Lifestyle changes, plus consider aspirin 75mg daily |
| Secondary thrombosis prevention | Venous event: Lifelong warfarin (INR range 2–3) Arterial event: Lifelong clopidogrel or warfarin (INR range 2–3) |
| Catastrophic antiphospholipid syndrome (CAPS) | Anticoagulation, intravenous corticosteroids, intravenous immunoglobulin and rituximab |
| Pregnant patients or patients who are considering becoming pregnant | Clinical criteria obstetric: Consider aspirin 75mg daily, plus prophylactic unfractionated heparin/low molecular weight heparin |
No treatment is recommended for patients who have aPL detected incidentally[3]
. However, modifiable factors that can increase the risk of thrombosis should be adjusted, (e.g. smoking, obesity and avoiding hormone replacement therapy or combined oral contraceptive[3]
). There is controversy regarding the use of aspirin for primary thromboprophylaxis as the risk of thrombosis is as low as 0–3.8% per year[5]
. Studies have also failed to show additional benefit in comparison with placebo[6]
, however, aspirin can be considered for higher risk patients with high levels of aPL[6]
. Primary thromboprophylaxis in this clinical setting is purely empirical.
Warfarin at an INR range of 2–3 (standard intensity) is recommended by the British Committee for Standards in Haematology (BCSH) for patients who present with venous thromboembolism (VTE)[11]
. The duration of treatment may be as little as three months in patients with episodes provoked by modifiable risk factors or continued for longer based on the risk/benefit as for individual patients without APS[11]
. Unproven thrombotic events (e.g. spontaneous proximal cerebral venous thrombosis or PE) carry a high risk of recurrence, regardless of the presence or absence of aPL or other thrombophilia. However, screening for aPL is recommended if anticoagulation would otherwise be discontinued (this should be undertaken at least seven days after stopping anticoagulation to allow coagulation to normalise[3]
). Anticoagulation can also interfere with the accuracy of LA testing[1]
. If aPL are detected, the risk of recurrence is probably higher, and long-term anticoagulation may be indicated[3]
. In patients with recurrent VTE on warfarin at standard intensity, a more aggressive approach with higher intensity (INR of 3–4) warfarin can be considered[5]
.
Patients who are aPL carriers and suffer stroke (unrelated to atrial fibrillation) are considered to be at high risk of recurrence and mortality, therefore, indefinite antithrombotic therapy is indicated[3]
. Routine aPL screening is not recommended for patients with stroke, unless they are younger (<50 years) ischaemic stroke patients[3]
. Treatment can include either antiplatelet therapy or warfarin, as both have been shown to be equally as effective[3]
. The BCSH recommends warfarin as the preferred choice in patients aged <50 years with triple-positive aPL, on account of risk of recurrence[3]
. However, antiplatelet therapy would be preferable in patients who are single aPL positive, based on the grounds of convenience[3]
. There is debate regarding the optimal treatment for APS patients with arterial disease[6]
.
Treatment during pregnancy
Managing APS in pregnancy is the most important factor for preventing recurrent miscarriages. The Royal College of Obstetrics and Gynaecologists recommend aspirin 75mg daily plus heparin because this combination leads to increases in live birth rates by approximately 50%[4]
. However, it is important to note that these trials had a number of weaknesses[12]
[13]
. Aspirin is recommended to be initiated preconception to have beneficial effect in the early stages of implantation[5]
. Close antenatal surveillance is also recommended[4]
. Thromboprophylaxis for seven days post-partum should also be considered[4]
.
Heparin is the anticoagulant of choice in this patient group because of its large molecular weight and polarity that prevents it from passing through the placenta[14]
. Even though there is no risk of teratogenicity or foetal haemorrhage, expected maternal risks are bleeding, hypersensitivity reactions, heparin-induced thrombocytopenia (HIT) and osteopenia[4]
. When comparing efficacy and safety profiles of unfractionated heparin (UFH) and low molecular weight heparin (LMWH), there is no significant difference in patient outcomes in APS[4]
. However, LMWH has potential advantages over UFH, such as once-daily dosing, and reduced incidence of HIT and heparin-induced osteoporosis. Heparin reverses the aPL effect on the trophoblasts as well as complement pathway[4]
.
A treatment dose (based on pre-pregnancy weight) of UFH/LMWH is recommended in women who have previously been maintained on long-term anticoagulation or have recently suffered a thrombotic event[5]
. A prophylactic dose would be more appropriate for women who meet the clinical criteria as outlined in box 1 for obstetric APS[6]
. Warfarin should be avoided in view of teratogenicity[6]
.
Other regimens include corticosteroids and intravenous immunoglobulins (IVIG), however, these are not routinely recommended; studies have shown that these do not improve live birth rate in APS, and also may contribute to maternal and foetal morbidity[4]
.
Current CAPS treatments
Treatment for CAPS is usually empirical, with the aim of improving survival rate. Therapies that have been used are: anticoagulation, plasmaphaeresis, IVIG, corticosteroids and rituximab[3]
. CAPS is listed as a grey indication for IVIG because of limited evidence by the Department of Health[15]
.
Future treatment considerations
Although antithrombotic therapy is the mainstay treatment for APS, there is still a need for well-designed trials to ensure optimal management. This is to avoid risks of disease-related thrombosis, as well as risks of medication-related haemorrhage. This is further complicated by the wide variability of patients having single-to-triple aPL detection to the different clinical presentations. Further studies are currently being carried out to investigate other drug therapies, such as statins[6]
, hydroxychloroquine[6]
and direct oral anticoagulants[16]
.
Jagjot K Chahal is cardiac and haematology pharmacist, Barts Health NHS Trust. Paul Wright is lead cardiac pharmacist, Barts Health NHS Trust. Peter MacCallum is senior lecturer in haematology, Barts and The London School of Medicine and Dentistry, Queen Mary University of London and honorary consultant haematology, Barts Health NHS Trust. Sotiris Antoniou is consultant pharmacist, cardiovascular medicine, Barts Health NHS Trust. Correspondence to: jagjot.chahal1@nhs.net
Financial and conflicts of interest disclosure:
The author has no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilised in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Miyakis S, Lockshin MD, Atsumi T et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;(4):295–306. doi: 10.1111/j.1538-7836.2006.01753.x
[2] Andreoli L, Chighizola CB, Banzato A et al. The estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis. Arthritis Care Res (Hoboken) 2013. doi: 10.1002/acr.22066
[3] Keeling D, Mackie I, Moore GW et al. Guidelines on the investigation and management of antiphospholipid syndrome. British Journal of Haematology, 2012;(157):47–58. doi: 10.1111/j.1365-2141.2012.09037.x
[4] Royal College of Obstetricians & Gynaecologists. The investigation and treatment of couples with recurrent first-trimester and second-trimester miscarriage. Green–top Guideline No. 17 April 2011. Available at: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_17.pdf (accessed June 2016).
[5] Mehdi A, Uthman I, Khamashta M et al. Treatment of antiphospholipid antibody syndrome. International Journal of Clinical Rheumatology 2010;5(2):241-254. doi: 10.2217/ijr.10.8
[6] Ruiz-Irastorza G, Crowther M, Branch W et al. Antiphospholipid syndrome. The Lancet 2010;(376):1498–1509. doi: 10.1016/S0140-6736(10)60709-X
[7] Wisloff F, Jacobsen EM & Liestol S. Laboratory diagnosis of the antiphospholipid syndrome. Thromb Res 2002;108:263–271. doi: 10.1016/s0049-3848(02)00400-0
[8] Tenedios F, Erkan D & Lockshin MD. Cardiac manifestations in the antiphospholipid syndrome. Rheumatic Disease Clinics of North America 2006;32(3):491-507. doi: 10.1016/j.rdc.2006.05.008
[9] Lim W, Crowther MA & Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006;295(9):1050–1057. doi: 10.1001/jama.295.9.1050
[10] Ruiz-Irastorza G, Hunt BJ & Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum 2007;57(8):1487–1495. doi: 10.1002/art.23109
[11] Keeling D, Baglin T, Tait C et al. Guidelines on oral anticoagulation with warfarin – fourth edition. British Journal of Haematology 2011;154(3):311-324. doi: 10.1111/j.1365-2141.2011.08753.x
[12] Kaandorp SP, Goddijn M, van der Post, JAM et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med 2010;362:1586–1596. doi: 10.1056/nejmoa1000641
[13] Clark P, Walker ID, Langhorne P et al. Scottish Pregnancy Intervention Study (SPIN) collaborators. SPIN (Scottish Pregnancy Intervention) study: a multicentre, randomised controlled trial of low-molecular-weight heparin and low-dose aspirin in women with recurrent miscarriage. Blood 2010;115(21):4162–4167. doi: 10.1182/blood-2010-01-267252
[14] Datta S. Anesthetic and obstetric management of high risk pregnancy, 3rd edition. Springer. 2004.
[15] Department of Health. Clinical guidelines for immunoglobulin use, 2nd edition. July 2011. Available at: https://www.gov.uk/government/publications/clinical-guidelines-for-immunoglobulin-use-second-edition-update (accessed June 2016).
[16] Betancur JF, Bonilla-Abadia F, Hormaza AA et al. Direct oral anticoagulants in antiphospholipid syndrome: a real life case series. Lupus 2016;25(6):658-662. doi: 10.1177/0961203315624555