Shutterstock.com
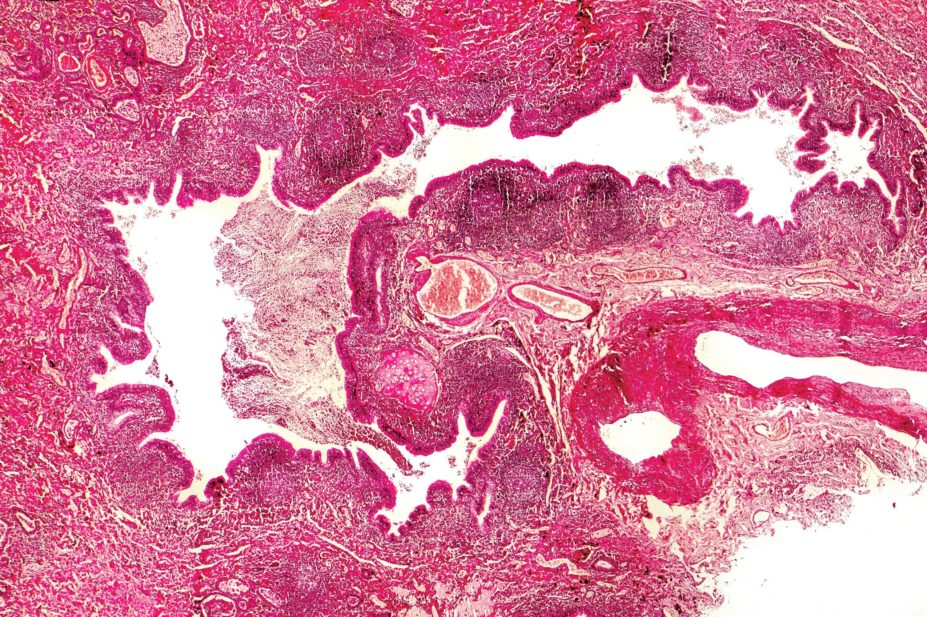
Bronchiectasis is the term given to abnormal irreversibly dilated and thickened bronchial walls. It is associated with recurrent cough and sputum production, and recurrent chest infections. Bronchiectasis is caused by repeated insults to the lower respiratory tract, altering muco-ciliary clearance that leads to stasis of secretions, infection, inflammation and, eventually, destruction and dilatation of the peribronchial and bronchial tree. The anatomical distribution of bronchiectasis may suggest an underlying aetiology. Upper lobe bronchiectasis may suggest cystic fibrosis (CF)-related bronchiectasis, allergic bronchopulmonary aspergillosis (ABPA), sarcoidosis or tuberculosis. A localised distribution of bronchiectasis suggests external compression, retained foreign body or even past pneumonia (see Table 1)[1]
. CF-related bronchiectasis has a different pathophysiology, therefore, its management is different and not covered in this article.
| Table 1: Main aetiologies of bronchiectasis | |
|---|---|
| Condition | Example |
| Genetic disorders | Cystic fibrosis, primary ciliary dyskinesia, alpha-1-antitrypsin deficiency, Young’s syndrome |
| Congenital | Bronchomalacia |
| Post-infectious | Mycobacteria (post-tuberculosis), pneumonia (bacterial, viral, fungal) |
| Iatrogenic | Post-chemotherapy/radiotherapy |
| Immune deficiency | HIV, common variable immune deficiency, antibody deficiency |
| Exaggerated immune response | Allergic bronchopulmonary aspergillosis |
| Mechanical | Foreign body, extrinsic compression, endobronchial lesions |
| Gastrointestinal disorders | Gastroesophageal reflux disease, inflammatory bowel disease |
| Connective tissue disorders | Rheumatoid arthritis, systemic lupus erythematosus, Sjogren’s syndrome, sarcoidosis, ankylosing spondylitis |
| Airways obstruction | Asthma, chronic obstructive pulmonary disease (COPD) |
| Idiopathic | No specific cause identified |
Epidemiology
Reported cases of bronchiectasis vary worldwide. In the UK, the point prevalence in 2013 in women was 566.1 per 100,000 individuals and 485.5 per 100,000 in men, with an increasing prevalence with age (more than 60 years old). There is around a two-fold increased mortality compared with the general population[2]
. In practice, life expectancy will be dependent on the severity of bronchiectasis and likely to be unaffected in patients with mild bronchiectasis, but reduced in those with severe bronchiectasis.
Diagnosis
Clinical presentation or severity of bronchiectasis in a patient can be variable. Patients with bronchiectasis usually present with chronic sputum production and recurrent chest infections. Some may also have haemoptysis, pleuritic chest pain, breathlessness, lethargy and weight loss. Examination findings include any or all of: pulmonary crackles, finger clubbing, cachexia, respiratory failure and cor-pulmonale. Radiological confirmation is required with a computed tomography (CT) of the chest. This also helps assess the severity of bronchiectasis (cylindrical, varicose and cystic), as well as the possible aetiology. Overall, this is defined as clinically significant bronchiectasis. In practice, mild radiological bronchiectasis can be identified on CT scan of the chest but if there are no symptoms suggestive of bronchiectasis, no action is needed unless clinically significant bronchiectasis ensues.
Microbiology
Patients with more advanced bronchiectasis are frequently infected with potential pathogenic microorganisms. Haemophilus influenzae is the commonest pathogen isolated in bronchiectasis. Pseudomonas
aeruginosa, Streptococcus pneumoniae, Staphylococcus aureus and Moraxella catarrhalis are also commonly isolated. In a study of 385 stable patients with bronchiectasis, pathogenic microorganisms were isolated in the baseline sputum cultures from 75.3% of patients. Of those with positive cultures, predominant organisms isolated were H. influenzae (38.6%); P. aeruginosa (21%); S. aureus (12.4%); M. catarrhalis (11.4%); S. pneumoniae (9.7%); and others (primarily enteric Gram-negative organisms, 9.3%)[3]
. The presence of S. aureus may be indicative of bronchiectasis due to CF, post-tuberculosis and post-ABPA. More than one organism may be isolated over time. There is increased interest in the potential for cross-infection, particularly with patients infected with P. aeruginosa. A small study by Mitchelmore et al. found evidence of cross-infection between three of 46 patients within an unsegregated bronchiectasis cohort. Longitudinal surveillance is warranted[4]
.
Non-tuberculous mycobacterium (NTM) is being isolated more often (around 9%) from patients with bronchiectasis, therefore, cultures for these organisms should be performed, especially in the case of unexplained deterioration. As these organisms are mainly environmental, to attribute the organism for the clinical deterioration, there should be at least two positive mycobacterial cultures or a single culture from invasive sampling (e.g. broncho-alveolar lavage)[5]
[6]
. NTM is known to cause both deteriorating nodular bronchiectasis and fibro-cavitatory disease. There are a variety of mycobacteria that have been implicated in bronchiectasis, with Mycobacterium avium complex being the most frequently identified mycobacterium to date.
The isolation of Candida species from sputum cultures usually indicates prior antibiotic treatment and does not require anti-fungal treatment. The isolation of Aspergillus fumigatus is seen in patients with ABPA and those taking long-term antibiotics.
Investigations
Initial investigations recommended in patients suspected of having bronchiectasis are aimed at establishing the underlying cause and assessing disease severity. Sputum is routinely tested for microbial culture and sensitivity, as well as mycobacterial culture. Relevant initial blood tests include full blood count, C-reactive protein and erythrocyte sedimentation rate (ESR) to assess the degree of systemic inflammation and whether there is peripheral blood eosinophilia. Depending on the suspected cause, other investigations may be necessary. Immunoglobulins (e.g. IgA, IgM and IgG, including IgG subclasses 1–4) are checked if immunoglobulin deficiency is suspected. Antibody levels to tetanus toxoid, S. pneumoniae and H. influenzae type B are assessed if antibody deficiency is suspected. Owing to the association with connective tissue disorders, anti-CCP, ANA, ANCA and anti-DS DNA can be tested. Finally, if allergic bronchopulmonary aspergillosis is suspected, the following investigations including serum IgE, skin prick testing or serum IgE testing to A. fumigatus and Aspergillus
precipitins need to be checked. If CF is suspected, a sweat test for chloride must be carried out, along with molecular blood tests for CFTR mutation analysis. If PCD is suspected, a nasal nitric oxide test should be carried out in the first instance.
Pulmonary function
Variable lung function tests can be seen in patients with bronchiectasis, even in people with more severe disease (some may have normal spirometry, a restrictive defect or airway obstruction). Patients with a forced expiratory volume in 1 second (FEV1) <30% predicted are at increased risk of hospitalisation and have a higher mortality[7]
.
At least annual FEV1 and forced vital capacity (FVC) and the FEV1/FVC ratio monitoring in patients is useful. In addition, monitoring spirometry post treatment with intravenous antibiotics is recommended as one of the markers of treatment response.
Radiology
Despite being quite non-specific, chest x-rays are often the initial mode of imaging used for investigating bronchiectasis. However, changes are only notable in severe disease, therefore, a normal chest x-ray does not exclude bronchiectasis. A high-resolution CT scan of the chest is considered the radiological investigation of choice. The signet sign seen on CT represents bronchial wall dilatation when the internal lumen’s diameter is greater than the adjacent pulmonary artery. Spiral and volumetric CTs have higher resolution compared with standard CT, but carry a higher radiation dose[8]
. CT scans can also assist in determining the cause of bronchiectasis such as ABPA (proximal changes), CF (upper lobe predominance), NTM (nodular bronchiectasis) and tracheobronchomegaly[9]
. The severity of bronchiectasis on CT also correlates with the level of airway obstruction on pulmonary function tests[10]
. Tubular bronchiectasis represents mild bronchiectasis, whereas varicose and cystic bronchiectasis represents more severe bronchiectasis. Having cystic bronchiectasis, or three or more lobes affected with bronchiectasis is used in the scoring system to assess risk of hospitalisation and mortality[7]
.
Bronchoscopy
If CT imaging shows localised disease, bronchoscopy is recommended to exclude an endobronchial lesion. Bronchoscopy with broncho-alveolar lavage is also useful in patients with suspected NTM infection who cannot produce regular sputum. In addition, bronchoscopy can help localise the site of bleeding in patients with haemoptysis and guide where to perform bronchial artery embolisation.
Treatment and goals
Symptom control, reduction in the number of exacerbations, preserving lung function and improving quality of life are the main aims of treatment in bronchiectasis. The British Thoracic Society (BTS) guidelines define an exacerbation as needing antibiotics and an acute deterioration with worsening symptoms (cough, increased sputum volume or change of viscosity, increased sputum purulence with or without increasing wheeze, breathlessness, haemoptysis) and/or systemic upset[6]
. Patient education on the disease goes a long way in helping with understanding of the disease, recognising exacerbations and compliance with treatment. Patients should all be provided a self-management plan; an example of which can be found on the BTS website[6]
.
The BTS guidelines recommend that certain patients with bronchiectasis should have regular follow up in secondary care in view of their poor prognosis[6]
. These include:
- Patients with recurrent exacerbations — three or more per year;
- Patients with deteriorating bronchiectasis;
- Patients with bronchiectasis associated with rheumatoid arthritis, immune deficiency, inflammatory bowel disease and primary ciliary dyskinesia;
- Patients with repeated isolates of NTM;
- Patients with methicillin-resistant S. aureus (MRSA) colonisation;
- Patients with chronic P. aeruginosa infection with two or more isolates, while stable in the last 12 months;
- Patients with ABPA;
- Patients on prophylactic antibiotic therapy;
- Patients with advanced disease and those requiring transplantation.
Physiotherapy
Physiotherapy is an essential part of treatment in bronchiectasis. It helps with expectoration of bronchopulmonary secretions and improves effective ventilation[11]
. Various airway clearance techniques can be used. In the UK, the active cycle breathing technique is the commonest technique, sometimes used in combination with postural drainage and manual techniques (see Table 2)[12]
. Patients are encouraged to be as independent as possible in doing their own physiotherapy. In case of an exacerbation when it may be difficult for the patient to carry out routine exercises, manual techniques can be considered for airway clearance. Adjuncts to airway clearance, such as 0.9% saline or hypertonic saline, can also be helpful before starting physiotherapy as they decrease sputum viscosity and increase the ease to expectorate[13]
. Lung function (in particular FEV1) should be checked before and after taking hypertonic saline because patients can develop bronchospasm. The duration and frequency of physiotherapy depends on the severity of bronchiectasis. In more advanced bronchiectasis, it should be carried out at least twice daily and sessions normally limited to 30 minutes per session. Currently there is very little information about the efficacy of non-invasive ventilation (NIV) in non-CF-bronchiectasis. In routine practice, NIV can be used in acute exacerbations in patients with hypercapnic respiratory failure.
| Table 2: Showing physiotherapy techniques used for airway clearance in bronchiectasis | |
|---|---|
| Method | Description |
| Postural drainage | This method uses gravity to move mucus from the deeper parts of the lung to the upper airway to facilitate expectoration |
| Autogenic drainage | This technique aims to increase expiratory airflow. Patients breathe at different lung volumes to loosen secretions and mobilise it towards larger airways |
| Positive expiratory pressure devices | This technique involves devices, such as flutter valve, acapella and RC cornet |
| High frequency chest wall oscillation | An inflatable vest performs chest physiotherapy by vibrating at a high frequency, which allows the patient to cough up secretions that have travelled to the larger airways |
Nutrition
A nutritional assessment should be made in patients with severe bronchiectasis because they are at risk of malnutrition. During each review, the patient’s body mass index (BMI) should be checked. A BMI of less than 18.5kg/m2 or weight loss of more than 5% in eight weeks or 10% in six months is evidence of malnutrition[14]
. A low BMI of less than 18.5kg/m2 is associated with poorer outcome[15]
.
Pharmacotherapy
Antibiotics for exacerbations
Several studies have shown that improved bacterial clearance is seen in patients who receive targeted antibiotics. Therefore, before starting antimicrobial therapy, a sputum sample should be sent for culture and sensitivity. If no microbiology is available, BTS guidelines recommend patients should initially be started on amoxicillin 500mg three-times daily (TDS) oral or clarithromycin 500mg twice daily (BD) oral (if they are allergic to penicillin)[6]
. If patients are not responding to the empirical antibiotics, the sputum culture results and in vitro sensitivity test results should be reviewed. Higher doses of prolonged antibiotics are needed in patients who have purulent sputum and severe bronchiectasis[16]
. Intravenous antibiotics are reserved for patients who have not responded to oral antibiotics, require hospital admission or have resistant pathogens (e.g. P. aeruginosa) that necessitate intravenous antibiotics. However, there are no randomised control trials (RCTs) that have explored the optimal duration of antibiotics. The BTS guidelines recommend 14 days for exacerbations because it is well recognised, particularly in more severe bronchiectasis, that prolonged and high antibiotic doses are needed[7]
. Common antibiotics used are shown in Table 3.
| Table 3: Antibiotics recommended for use in exacerbations | ||
|---|---|---|
| Organism | Recommended first-line treatment | Recommended second-line treatment |
| Streptococcus pneumoniae | Amoxicillin 500mg three-times daily (TDS) | Doxycycline 100mg twice daily (BD) |
| Haemophilus influenzae— beta lactamase negative | Amoxicillin 500mg TDS Or Amoxicillin 1g TDS Or Amoxicillin 3g BD | Doxycycline 100mg BD Or Ciprofloxacin 500mg or 750mg BD Or Ceftriaxone 2g once daily (OD) intravenous |
| Haemophilus influenzae -beta lactamase positive | Amoxicillin with clavulanic acid 625mg one tablet TDS | Doxycycline 100mg BD Or Ciprofloxacin 500mg or 750mg BD Or Ceftriaxone 2g OD intravenous |
| Moraxella catarrhalis | Amoxicillin with clavulanic acid 625mg one tablet TDS | Clarithromycin 500mg BD Or Doxycycline 100mg BD Or Ciprofloxacin 500mg or 750mg BD |
| Methicillin-susceptible Staphylococcus aureus (MSSA) | Flucloxacillin 500mg four times daily (QDS) | Clarithromycin 500mg BD Or Doxycycline 100mg BD Or Amoxicillin with clavulanic acid 625mg one tablet TDS |
| Methicillin-resistant Staphylococcus aureus (MRSA) Oral preparations | Doxycycline 100mg BD
Rifampicin (<50kg) 450mg OD Rifampicin (>50kg) 600mg OD
Trimethoprim | Third-line: Linezolid 600mg BD |
| MRSA Intravenous preparations | Vancomycin 1gm BD* (monitor serum levels and adjust dose accordingly) Or Teicoplanin 400mg OD | Linezolid 600mg BD |
| Coliforms (e.g. Klebsiella, enterobacter) | Oral ciprofloxacin 500mg or 750mg BD | Intravenous Ceftriaxone 2g OD |
| Pseudomonas aeruginosa | Oral ciprofloxacin 500mg BD (750mg BD in more severe infections) | Monotherapy: Intravenous ceftazidime 2g TDS Or Piperacillin with tazobactam 4.5g TDS Or Aztreonam 2g TDS Or Meropenem 2g TDS
Combination therapy: The above can be combined with gentamicin or tobramycin or colistin 2 MU TDS (<60kg; 50,000–75,000 units/kg daily in three divided doses) |
| Caution with aminoglycosides in pregnancy, renal failure, the elderly or those on multiple other medicines. *Elderly (>65 years), 500mg vancomycin every 12 hours or 1g OD Source: BNF 72 | ||
The use of adjunct nebulised tobramycin given in combination with oral ciprofloxacin during exacerbations was more effective at reducing the bacterial load compared with oral ciprofloxacin alone, but did not improve clinical outcomes, which likely reflects the increased local side effects with inhaled tobramycin[17]
.
Combination antibiotics may be required in some circumstances but are normally not required for H. influenzae, M. catarrhalis, S. pneumoniae or S. aureus (methicillin sensitive). If there are multiple organisms grown on sputum culture, choosing an antibiotic that covers all these organisms is preferred[7]
. In drug-resistant pathogens, there is no evidence that combination antibiotics leads to improved clinical outcomes, however, it is thought to reduce the risk of further drug resistance developing[6]
.
Long-term antibiotics
According to BTS guidelines, these are considered in patients who have three or more exacerbations in a year requiring antibiotics[7]
. If patients are not having multiple exacerbations, but recover slowly from exacerbations, they can also be considered for long-term antibiotic therapy.
Macrolides: The BLESS trial was a double-blind, randomised, placebo-controlled trial that assessed the effect of long-term, low-dose erythromycin (erythromycin ethylsuccinate; 400mg, which is equivalent to 250mg erythromycin base) BD on exacerbations of bronchiectasis. The primary outcome was the reduction in annual mean rate of protocol defined pulmonary exacerbations per patient. In total, 117 patients were recruited and randomised to either the erythromycin group (n=59) or the placebo group (n=58). The study demonstrated a significant reduction in the number of exacerbations in the erythromycin group compared with the placebo group overall and a pre-specified subgroup with baseline P. aeruginosa infection. The erythromycin group also showed a reduction in sputum production over 24 hours and attenuation of lung function decline. However, there was an increased rate of macrolide resistance in the erythromycin group[18]
.
The BAT trial assessed the efficacy of daily oral azithromycin maintenance treatment on exacerbations of non-CF bronchiectasis during one year of treatment. Overall, 43 patients were randomised to receive 250mg of azithromycin daily, while 40 patients received placebo. The study concluded that patients on azithromycin 250mg once daily for a year compared with placebo had fewer exacerbations during treatment, showed improved FEV1 and FVC and quality of life. However, the sputum microbiology remained similar[19]
.
The EMBRACE trial assessed azithromycin 500mg three times a week for six months and showed a reduction in overall exacerbations at the end of treatment and six months post-treatment. Azithromycin is thought to have bacteriostatic and anti-inflammatory effects by decreasing the production of pro-inflammatory cytokines[20]
. Patients on long-term macrolides may have experienced side effects such as tinnitus, hearing impairment, cholestatic hepatitis, gastrointestinal problems and possibly increased vascular events. The long-term impact of increased macrolide resistance with the oropharyngeal flora needs to be established.
Other long-term antibiotics: A systematic review on inhaled/nebulised antibiotics from 28 days to one year showed that they may provide an effective bacterial suppressive therapy with an acceptable safety profile in adult patients with stable non-CF bronchiectasis and chronic bronchial infection[21]
.
The efficacy of nebulised gentamicin over 12 months in non-CF bronchiectasis was assessed in a RCT published in 2011. In total, 65 patients were randomised to either 80mg BD of nebulised gentamicin or placebo (nebulised saline 0.9%). The gentamicin group were found to have reduced bacterial density compared with the saline group, as well as 30.8% eradication in those infected with P. aeruginosa and 92.8% with other pathogens. The nebulised gentamicin group were also found to have less sputum purulence (p<0.0001), greater exercise capacity (p=0.03) and fewer exacerbations (p<0.0001) with increased time to first exacerbation. In terms of quality of life, the gentamicin group showed greater improvements in their St. George’s Respiratory questionnaire (p<0.004)[22]
.
Two randomised, double-blind, placebo-controlled phase III trials assessed the safety and efficacy of nebulised aztreonam. The trials (AIR-BX1 and AIR-BX2) included patients who had bronchiectasis with Gram-negative organisms from sputum or bronchoscopic samples. Patients were randomised to receive aztreonam for inhalation solution (AZLI) 75mg TDS or placebo TDS. In both studies, two 4-week courses of nebulised AZLI 75mg or placebo were each followed by a 4-week off-treatment period. In AIR-BX1, 134 patients received AZLI and 132 received placebo. In AIR-BX2, 136 patients received AZLI, while 138 patients received placebo. The primary end point was change from baseline of the quality of life-bronchiectasis respiratory symptoms score at 4 weeks. AZLI did not provide significant clinical benefit as a treatment for non-CF bronchiectasis because there was insignificant improvement in the primary end point and there were more adverse events, in particular increased dyspnoea, cough and sputum production[23]
.
Nebulised tobramycin has been investigated for use in patients with bronchiectasis and chronic infection with P. aeruginosa. A small crossover study, published in 2005, was carried out to assess the clinical effectiveness and safety of nebulised tobramycin therapy. Patients (n=30) were given 300mg nebulised tobramycin or placebo BD for six months, followed by a one month washout period before receiving the other therapy. With nebulised tobramycin, there was a reduction in P. aeruginosa density in sputum, a reduction in risk of hospitalisation and number of days of hospitalisation. Overall, 10% of patients stopped treatment due to bronchospasm[24]
.
The outcomes of the one-year studies with inhaled ciprofloxacin and nebulised ciprofloxacin are yet to be published.
Licensed inhaled/nebulised antibiotics for patients with bronchiectasis are urgently needed; in the UK nebulised gentamicin, colomycin and tobramycin are all used, but are currently unlicensed.
Antimicrobials for NTM
For patients who require treatment, it is usually for 18–24 months, and in the UK triple therapy is given (rifampicin plus ethambutol and clarithromycin/ciprofloxacin/moxifloxacin). In macrolide-resistant organisms, an alternative regimen could be used such as rifampicin, ethambutol and moxifloxacin. Inhaled amikacin may be a useful adjunct in poorly responding patients to enhance mycobacterial clearance.
Allergic bronchopulmonary aspergillosis (ABPA)
For active ABPA, treatment is with oral corticosteroids. An initial dose of prednisolone 0.5mg/kg/d, for 1–2 weeks, is recommended, before weaning by 5–10mg every 2 weeks until discontinuing according to clinical response and serum IgE levels. Itraconazole is used as a steroid-sparing agent for patients dependent on oral corticosteroids where difficulty in weaning is experienced. In addition, removal of environmental exposure of A. fumigatus spores is recommended (e.g. renovation, construction, mucking out stables).
Mucolytics
The only study in bronchiectasis where there is sufficient evidence for mucolytics is for nebulised DNase, which is essentially a mucus-degrading agent. The BTS guidelines on bronchiectasis recommend not using DNase because the previous RCT in 349 patients showed increased exacerbations and an accelerated fall in FEV1 for those taking nebulised DNAse for 24 weeks[6],[25]
.
Hyperosmolar agents
These agents are used to improve hydration of the airway surface and mucociliary clearance. The hyperosmolar agents that we have most evidence for are hypertonic saline and mannitol. The study by Nicolson and colleagues showed similar efficacy with 0.9% saline and hypertonic saline[26]
. However, further studies are required to show whether saline (0.9% or hypertonic) is recommended in practice. Bilton et al. studied the effects of inhaled mannitol in a 12-month, double-blinded RCT. There was no statistically significant reduction in exacerbation rates. There was, however, an improvement in time to first exacerbation and quality of life[27]
.
Bronchodilators
Bronchodilators are often used to treat more advanced bronchiectasis, especially where patients also have co-existing asthma or COPD. Bronchodilators improve ciliary motility and facilitate clearance of secretions, and they are sometimes used before physiotherapy. Beta agonists and anticholinergics are often recommended for patients with breathlessness, however, no trials have assessed the effect of them in patients with bronchiectasis. If there is benefit with short-acting bronchodilators, long-acting bronchodilators are used.
Inhaled corticosteroids (ICS)
A double-blind, placebo-controlled trial showed that ICS, in the form of fluticasone, are associated with reduced neutrophil count and inflammatory markers such as interleukins-1,8 and Leukotriene B4[28]
. Martinez-Garcia et al. in their prospective, randomised, double-blind study investigated whether ICS improve quality of life. Overall, 93 patients were randomised to receive either fluticasone 250µg BD, fluticasone 500µg BD or no treatment for 6 months. Patients who received 500µg BD of fluticasone had better symptom control from the first month of treatment according to their health-related questionnaire scores[29]
. However, to date, RCTs have not shown a reduction of exacerbations. ICS are recommended by the BTS guidelines in patients with co-existing asthma and COPD, if they meet the criteria for ICS for asthma or COPD[6]
.
Combination inhaled therapy
A double-blind, randomised, parallel group clinical study of non-CF bronchiectasis patients (n=40) evaluated the efficacy and safety of medium-dose formeterol–budesonide (18/640µg daily) combination inhaler compared with a high-dose budesonide (1600µg daily) treatment over a one-year period. The formoterol–budesonide combination therapy was found to be more efficacious and safe in terms of symptom control, improvement in quality of life and fewer side effects than high-dose ICS alone[30]
. There is also a theoretical increased risk of pneumonia and NTM infection in patients with chronic respiratory disease, such as bronchiectasis on ICS[31],[32]
.
Vaccination
There have been no specific RCTs on vaccines for patients with bronchiectasis. However, patients should still be advised to have the influenza vaccine on an annual basis and pneumococcal polysaccharide vaccine (PPV-23) if they have not had it previously.
Surgery
Patients who demonstrate unilateral localised disease and are not improving with maximal medical therapy should consider resection surgery[33]
. Patients who have massive haemoptysis should also consider surgery. Currently, bronchial artery embolisation is the first-line treatment and surgery regarded as second line. Bilateral lung transplantation should be considered in patients aged 60 years and under with end-stage disease and predicted FEV1 of <30% if they continue to deteriorate despite medical therapy. Five-year survival is thought to be around 55–60%[34]
. In most patients, double lung transplant is preferred to single lung transplant because the transplanted lung may become infected by pathogens that have colonised the diseased lung.
End-of-life care
Long-term oxygen therapy should be considered in bronchiectasis patients who develop respiratory failure. Along with active treatment, it is important to recognise when a patient with end-stage bronchiectasis is approaching end of life. Involvement of the palliative care team and planning for an advance directive are useful in symptom control and respecting the patient’s wishes.
Future perspective
Management of bronchiectasis will benefit from national and international networks and consensus guidelines, such as the recently published European Respiratory Society guidelines[35]
on bronchiectasis, and the, soon to be published, updated BTS guidelines[6]
[35]
. There have been BTS quality standards in bronchiectasis, which aim to improve care throughout the UK and, if adopted, internationally. Therefore, it will be interesting whether guidelines, quality standards and self-management plans lead to qualitative or quantitative improvement. Owing to the difficulties in treating bronchiectasis and the absence of large randomised trials, there is an urgent need for licensed therapies, in particular, long-term antibiotics, long-term anti-inflammatory treatment and muco-active therapies.
Financial and conflicts of interest disclosure:
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was used in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Chalmers JD, Aliberti S, Blasi F. State of the art review: management of bronchiectasis in adults. Eur Respir J 2015;45:1446–1462. doi: 10.1183/09031936.00119114
[2] Quint JK, Millet ER, Joshi M et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016;47(1):186–193. doi: 10.1183/13993003.01033-2015
[3] James DC, Maeve PS, Brian JM et al. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non–cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012;186:657–665. doi: 10.1164/rccm.201203-0487OC
[4] Mitchelmore PJ, Randall J, Bull J et al. Molecular epidemiology of Pseudomonas aeruginosa in an unsegregated bronchiectasis cohort sharing hospital facilities with a cystic fibrosis cohort. Thorax 2017; Online ahead of print. doi: 10.1136/thoraxjnl-2016-209889
[5] Chu H, Zhao L, Xiao H et al. Prevalence of non-tuberculous mycobacterium in patients with bronchiectasis: a meta-analysis. Arch Med Sci 2014;10(4):661–668. doi: 10.5114/aoms.2014.44857
[6] Pasteur MC, Bilton D, Hill AT. Guideline on non-CF bronchiectasis. Thorax 2010;65 Suppl 1:i1–58. doi: 10.1136/thx.2010.136119
[7] Chalmers JD, Geominne P, Aliberti S et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189(5):576–585. doi: 10.1164/rccm.201309-1575OC
[8] Lucidarme O, Grenier P, Coche E. Bronchiectasis: comparative assessment with thin-section CT and helical CT. Radiology 1996;200(3):673–679. doi: 10.1148/radiology.200.3.8756913
[9] Cartier Y, Kavanagh PV, Johkoh T. Bronchiectasis: accuracy of high-resolution CT in differentiation of specific diseases. Am J Roentgenol 1999; 173:47–52. doi: 10.2214/ajr.173.1.10397098
[10] Roberts HR, Well AU, Milne DG et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax 2000;55:198–204. doi: 10.1136/thorax.55.3.198
[11] Bott J & Moran F. Physiotherapy and NIPP, Ch. 14. In: Simonds AK. (ed) Non-Invasive Ventilatory Support, London: Chapman & Hall, 1996. p. 133–142
[12] O’Neill B, Bradley JM, McArdle N et al. The current physiotherapy management of patients with bronchiectasis: a UK survey. Int J Clin Phar 2002;56(1):34–35. PMID: 11831830
[13] Patterson JE, Bradley JM, Hewitt O et al. Airway clearance in bronchiectasis: a randomised control trial of active cycle of breathing techniques versus Acapella. Respiration 2005;72(3):239–242. doi: 10.1159/000085363
[14] Vendrell M, Gracia J, Oliveira C. Diagnosis and treatment of bronchiectasis. Arch Bronchopeumol 2008;44(11):629–640. doi: 10.1016/s1579-2129(08)60117-2
[15] European Bronchiectasis Registry. EMBARC: Severity assessment. (Online). Available at: https://www.bronchiectasis.eu/severity-assessment (accessed November 2017)
[16] Hill SL, Morrison HM, Burnett D et al. Short term response of patients to treatment with amoxycillin given in standard or high doses orally or by inhalation. Thorax 1986;41(7):559–565. doi: 10.1136/thx.41.7.559
[17] Bilton D, Henig N, Morrissey B et al. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbation of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest 2006; 130(5):1503–1510. doi: 10.1378/chest.130.5.1503
[18] Serisier DJ, Martin ML, McGuckin MA et al. Effect of long term low dose erythromycin on pulmonary exacerbations among patients with non-CF bronchiectasis. JAMA 2013;309(12):1260–1267. doi: 10.1001/jama.2013.2290
[19] Altenburg J, de Graaff CS, Stienstra Y et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non cystic fibrosis bronchiectasis. JAMA 2013;309(12):1251–1259. doi: 10.1001/jama.2013.1937
[20] Wong C, Jayaram L, Karalus N et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomized, double-blind, placebo-controlled trial. Lancet 2012;380(9842):660–667. doi: 10.1016/S0140-6736(12)60953-2
[21] Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non-cystic fibrosis bronchiectasis: a systemic review. Eur Respir J 2014;44(2):382–393. doi: 10.1183/09031936.00018414
[22] Murray MP, Govan JR, Doherty CJ et al. A randomised control trial of nebulized gentamicin in non-CF bronchiectasis. Am J Respir Crit Care Med 2011;183(4):491–499. doi: 10.1164/rccm.201005-0756OC
[23] Barker AF, O’Donnell AE, Flume P et al. Aztreonam for inhalation solution in patients with non-CF bronchiectasis (AIR-BX1 and AIR-BX2): two randomized double-blind, placebo-controlled phase 3 trials. Lancet Repir Med 2014;2(9):738–749. doi: 10.1016/S2213-2600(14)70165-1
[24] Drobnic ME, Sune P, Montoro JB et al. Inhaled tobramycin in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection Pseudomonas aeruginosa. Ann Pharmacother. 2005;39(1):39–44. doi: 10.1345/aph.1e099
[25] O’Donnell A, Barker AF, Ilowite JS et al; rhDNase Study Group. Treatment of idiopathic bronchiectasis with aerolized recombinant human DNase I. Chest 1988;113(5):1329–1334. doi: 10.1378/chest.113.5.1329
[26] Nicolson CHH, Stirling RG, Borg BM et al. The long term effect of inhaled hypertonic saline 6% in non-cystic fibrosis bronchiectasis. Respir Med 2012;106:661–667. doi: 10.1016/j.rmed.2011.12.021
[27] Bilton D, Tino G, Barker AF et al. Inhaled mannitol for non-cystic fibrosis bronchiectasis: a randomised, controlled trial. Thorax 2014;69:1073–1079. doi: 10.1136/thoraxjnl-2014-205587
[28] Tsang KW, Ho PL, Lam WK. Inhaled fluticasone reduces sputum inflammatory indices in severe bronchiectasis. Am J Respir Crit Care Med 1998;158(3):723–727. doi: 10.1164/ajrccm.158.3.9710090
[29] Martinez-Garcia M, Perpina-Tordera M, Roman-Sanchaez P et al. Inhaled steroids improve quality of life in patients with steady state bronchiectasis. Resp Med 2006;100(9):1623–1632. doi: 10.1016/j.rmed.2005.12.002
[30] Martinez-Garcia M, Soler-Cataluna JJ, Catalan-Serra P et al. Clinical efficacy and safety of budesonide-formoterol in non-cystic fibrosis bronchiectasis. Chest 2012;141(2):461–468. doi: 10.1378/chest.11-0180
[31] Mayor ER, Serrano VA, Amara-Elori I et al. Chronic inhaled corticosteroids in patients with bronchiectasis who suffer an exacerbation. Eur Respir J 2016;48:PA1548. doi: 10.1183/13993003.congress-2016.PA1548
[32] AndreJak AC, Nielsen R, Thomsen V et al. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256–262. doi: 10.1136/thoraxjnl-2012-201772
[33] Bagheri R, Haghi SZ, Fattahi Masoum SH et al. Surgical management of bronchiectasis: analysis of 277 patients. Thorac Cardiovasc Surg 2010;58(5):291–294. doi: 10.1055/s-0030-1249941
[34] Weiss ES, Allen JG, Meguid RA. The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg 2009;88(4):1062–1070. doi: 10.1016/j.athoracsur.2009.06.005
[35] Polverino E, Goeminne PC, McDonnell MJ et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017;50:1700629. doi: 10.1183/13993003.00629-2017