Victor de Schwanberg / Science Photo Library / reproduced with permission from Gut 2017;66(9):1631–1
Cancer learning ‘hub’
Pharmacists are playing an increasingly important role in supporting patients with cancer, working within multidisciplinary teams and improving outcomes. However, in a rapidly evolving field with numbers of new cancer medicines is increasing and the potential for adverse effects, it is now more important than ever for pharmacists to have a solid understanding of the principles of cancer biology, its diagnosis and approaches to treatment and prevention. This new collection of cancer content, brought to you in partnership with BeOne Medicines, provides access to educational resources that support professional development for improved patientAetiology
Onset of CRC is influenced by a complex interaction between genetic, environmental and lifestyle factors, which include:- Fruit and vegetable intake[6] ;
- Alcohol consumption[7] ;
- Smoking status[8] ,[9] ;
- Red and processed meat consumption[10] ;
- Body mass index[11] ,[12] ;
- Exercise frequency[13],[14],[15],[16] ;
- Age[17] .

Symptoms
There are many symptoms of CRC that pharmacists should be aware of, including[21] :- A persistent change in bowel habit;
- Bleeding from the back passage;
- Blood in the stool;
- A lump in the abdomen;
- Anaemia;
- Unexplained tiredness;
- Weight loss;
- Abdominal pain.
The adenoma-carcinoma pathway
More than 90% of CRCs develop from adenomas (also referred to as adenomatous polyps), which are benign growths that develop from gland cells that line the bowel wall[22],[23],[24] . Around 33–50% of all adults will develop one or more adenomas during their lifetime[25],[26] and, while all have the potential to become malignant, fewer than 10% develop into invasive cancer[24] ,[27] . The likelihood that an adenoma will develop into invasive cancer is highly dependent on a number of factors, including: the size and histological type of the adenoma; the degree of epithelial dysplasia (i.e. the extent to which cells appear to be abnormal); and the involvement of specific tumour suppressor genes[28] (see Box).- Stage I
- Stage II
- Stage III
- Stage IV
Screening for colorectal cancer in the UK
Several investigations are capable of testing for CRC and the precancerous lesions from which it develops[35] . These can be broadly categorised into two groups:- Those that detect cancer early (e.g. the guaiac faecal occult blood test [FOBt] and the faecal immunochemical test [FIT]);
- Those that prevent cancer through the timely detection and removal of adenomas (e.g. flexible sigmoidoscopy and colonoscopy).
Bowel scope screening for men and women aged 55–59 years (England only)
Introduced in 2013, bowel scope screening is gradually being rolled out to all men and women aged 55–59 years who are registered with a GP in England. It involves an endoscopic test called a ‘flexible sigmoidoscopy’ that examines the rectum and sigmoid colon (the most distal third of the large bowel where cancer is most likely to develop) for adenomas and cancer. Patients are invited to a one-off test at the age of 55 years, however, patients are able to self refer up to their 60th birthday. This test has been shown to reduce CRC incidence among users by up to 33% and CRC mortality by up to 50%[36] .Faecal occult blood test screening for men and women aged 60–74 years (England, Wales and Northern Ireland)
This home-based screening test is offered once every two years to men and women aged 60–74 years who are registered with a GP in England, Wales and Northern Ireland[37] . People aged 75 years or older can ask for a kit every two years by calling the free bowel cancer screening helpline on 0800 7076060. Individuals complete the FOBt by applying a small stool sample onto a test card impregnated with guaiac (a resin that acts as phenolic redox indicator), using a cardboard applicator (see Figure 1). Two samples are usually taken from each bowel motion, so as to reduce the chances of obtaining a false negative result (i.e. a negative result for a sample that contains faecal occult blood). As tumours and adenomas can bleed intermittently, and the test may not be sensitive enough to detect particularly low concentrations of faecal haemoglobin[38] , the stool sampling process is repeated so that a sample is taken from up to three separate bowel motions. The test kit is returned by post to a screening centre for analysis[37] . If there is a positive result in five or six panels, the patient is referred to their local screening centre for suitability assessment for a colonoscopy[37] ,[39],[40] . If there is a positive result in one to four panels, another kit is sent to the patient’s home address for repeat testing.
Figure 1: Guaiac faecal occult blood test kit used by the NHS Bowel Cancer Screening Programme
Source: Victor de Schwanberg / Science Photo Library
Faecal immunochemical test screening for men and women aged 50–74 years (Scotland)
This home-based screening test is offered once every two years to men and women aged 50–74 years registered with a GP in Scotland. Similar to the FOBt, individuals completing the FIT are required to collect a sample of their stool. However, rather than applying a sample to a test card, it is stored in a bottle containing buffer solution using a plastic applicator (see Figure 2)[41] . As with the FOBt, the FIT is completed in the individual’s home and then returned to a screening centre where it is examined by a medical laboratory assistant using an analyser. A recent pilot study examining the use of FIT in England showed a 7% increase in uptake compared with the FOBt[42] . As a result, Public Health England (PHE) has recently confirmed that FIT will replace the FOBt in the English Bowel Cancer Screening Programme and is scheduled to be phased in spring 2019. There are also recommendations that the screening age of the English programme is to be lowered from 60 to 50 years, as is currently the case in Scotland[43] .
Figure 2: Instructions for the faecal immunochemical test kit piloted by the NHS Bowel Cancer Screening Programme
Source: Reproduced with permission from Gut 2017;66(9):1631–1644
Step 1: Write date on sample bottle; use layers of toilet paper to catch the sample; twist cap to open sample bottle. Step 2: Collect sample by scraping the green, stick along the sample until all grooves are covered. Step 3: Put stick back in and ‘click’ the green cap to close it; do not repeat the collection; wash hands after use. Step 4 Put the sample bottle back into the box; write your name on the box in the space provided; peel off tape, close and seal the box.Uptake of CRC screening
CRC screening uptake across the UK is lower than the other cancer screening programmes, and there are considerable inequalities between areas and demographics. PHE has an uptake target of 75% but the most recent uptake figure stands at 56%[44] . In some parts of the country, uptake is as low as 33% according to figures from Bowel Cancer UK. Seven out of ten of the worst areas in the country are in London and 44% of clinical commissioning groups in England are below the national average[44] .The role of community pharmacies
In 2016, PHE established the quality criteria for community pharmacies to gain Health Living Pharmacy Status and the role of health promotion in meeting public health needs. This was further defined in the National Institute for Health and Care Excellence guideline ‘Community pharmacies: promoting health and wellbeing’, published in August 2018[45] . Building a relationship with a local bowel cancer screening centre and collaborating on the promotion of CRC screening in the local population will assist community pharmacies in demonstrating how they are meeting the criteria laid out in these documents. For example, Healthy Living Pharmacies could ensure information, including the upcoming changes to the NHS Bowel Cancer Screening programme, is part of their Health Promotion Zones with relevant information on their local screening centre. Team leaders within community pharmacies could ensure all staff receive training on bowel cancer to include:- Providing up-to-date information – the facts about CRC;
- Benefits of early detection (including ‘red flag’ symptoms to look out for);
- The change to the FIT home testing kit;
- Ensuring staff feel confident on how to use the CRC testing kit and are able to explain it to patients in a simple and concise manner.
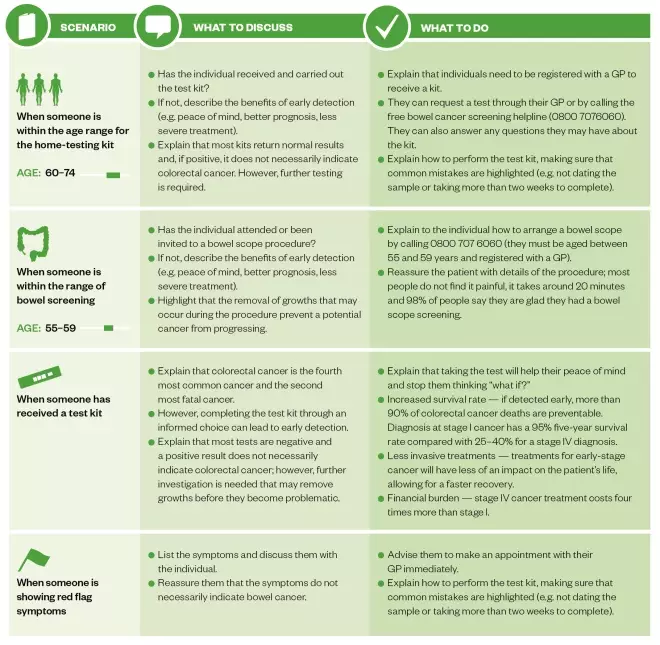
Table: Advice pharmacists can provide people concerned about colorectal cancer screening
Source: St Mark’s Hospital, Middlesex
References
[1] Cancer Research UK. Bowel cancer incidence statistics. 2018. Available at: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/incidence/uk-bowel-cancer-incidence-statistics (accessed January 2019)
[2] Cancer Research UK. Bowel cancer mortality statistics. 2018. Available at: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/mortality/ (accessed January 2019)
[3] Cancer Research UK. Cancer incidence for common cancers. 2018. Available at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#heading-Zero (accessed January 2019)
[4] Cancer Research UK. Cancer mortality for common cancers. 2018. Available at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/mortality/common-cancers-compared#heading-Zero (accessed January 2019)
[5] Office for National Statistics. Bowel cancer rates have risen in England since 1971. 2013. Available at: https://webarchive.nationalarchives.gov.uk/20140112045301/http://www.ons.gov.uk/ons/rel/vsob1/cancer-statistics-registrations–england–series-mb1-/no–41–2010/sty-bowel-cancer-rates-in-england.html?translation-component=&calling-id=77-6741-4&currLang=English&format=normal (accessed January 2019)
[6] van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2009;89(5):1441–1452. doi: 10.3945/ajcn.2008.27120
[7] Cho E, Smith-Warner SA, Ritz J et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Int Med 2004;140(8):603–613. PMID: 15096331
[8] Botteri E, Iodice S, Bagnardi V et al. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300(23):2765–2778. doi: 10.1001/jama.2008.839
[9] Liang PS, Chen TY & Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: Systematic review and metaâ€analysis. Int J Cancer 2009;124(10):2406–2415. doi: 10.1002/ijc.24191
[10] Chan D, Lau R, Aune D et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLOS One 2011;6(6):e20456 doi: 10.1371/journal.pone.0020456
[11] Moghaddam AA, Woodward M & Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidem Biomar 2007;16(12):2533–2547. doi: 10.1158/1055-9965.EPI-07-0708
[12] Ning Y, Wang L & Giovannucci E. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev 2010;11(1):19–30. doi: 10.1111/j.1467-789X.2009.00613.x
[13] Slattery ML. Physical activity and colorectal cancer. Sports Med 2004;34(4):239–252. PMID: 15049716
[14] Wei EK, Giovannucci E, Wu K et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer 2004;108(3):433–442. doi: 10.1002/ijc.11540
[15] Wolin K, Yan Y, Colditz G & Lee I. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer 2009;100(4):611–616. doi: 10.1038/sj.bjc.6604917
[16] Boyle T, Fritschi L, Heyworth J & Bull F. Long-term sedentary work and the risk of subsite-specific colorectal cancer. Am J Epidemiol 2011;173(10):1183–1191. doi: 10.1093/aje/kwq513
[17] Cancer Research UK. Bowel cancer incidence statistics by age. 2018. Available at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence#heading-One (accessed January 2019)
[18] Winawer SJ. Colorectal cancer screening. Best Pract Res Clin Gastroenterol 2007;21(6):1031–1048. doi: 10.1016/j.bpg.2007.09.004
[19] Canavan C, Abrams K & Mayberry J. Metaâ€analysis: Colorectal and small bowel cancer risk in patients with Crohn’s disease. Alimen Pharm Ther 2006;23(8):1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x
[20] Jess T, Rungoe C & Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol 2012;10(6):639–645. doi: 10.1016/j.cgh.2012.01.010
[21] Cancer Research UK. Bowel cancer symptoms. 2018. Available at: http://www.cancerresearchuk.org/about-cancer/type/bowel-cancer/about/bowel-cancer-symptoms (accessed January 2019)
[22] Winawer SJ & Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am 2002;12(1):1–9. PMID: 11916153
[23] Stryker SJ, Wolff BG, Culp CE et al. Natural history of untreated colonic polyps. Gastroenterology 1987;93(5):1009–1013. PMID: 3653628
[24] Risio M. The natural history of adenomas. Best Pract Res Cl Ga. 2010;24(4):397–406. doi: 10.1016/j.bpg.2010.04.005
[25] Bond JH. Polyp guideline: Diagnosis, treatment, and surveillance for patients with colorectal polyps. Am J Gastroentrol 2000;95(11):3053–3063. doi: 10.1111/j.1572-0241.2000.03434.x
[26] Schatzkin A, Freedman LS, Dawsey SM & Lanza E. Interpreting precursor studies: what polyp trials tell us about large-bowel cancer. J Nat Cancer Inst 1994;86(14):1053–1057. PMID: 7802771
[27] Levine JS & Ahnen DJ. Adenomatous polyps of the colon. N Eng J Med 2006;355(24):2551–2557. doi: 10.1056/NEJMcp063038
[28] Vogelstein B & Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004;10(8):789–799. doi: 10.1038/nm1087
[29] Cancer Research UK. Bowel cancer survival statistics. 2017. Available at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/survival#ref-0 (accessed January 2019)
[30] Komuta K, Furui J, Haraguchi M & Kanematsu T. The detection of colorectal cancer at an asymptomatic stage by screening is useful. Hepato-gastroenterol 2000;47(34):1011–1014. PMID: 11020866
[31] Verdecchia A, Guzzinati S, Francisci S et al. Survival trends in European cancer patients diagnosed from 1988 to 1999. Eur J Cancer 2009;45(6):1042–1066. doi: 10.1016/j.ejca.2008.11.029
[32] Cancer Research UK. Treatment decisions for early bowel cancer. 2015. Available at: http://www.cancerresearchuk.org/about-cancer/bowel-cancer/treatment/treatment-decisions (accessed January 2019)
[33] Cancer Research UK. Treatment for advanced cancer. 2018. Available at: http://www.cancerresearchuk.org/about-cancer/bowel-cancer/advanced/treatment (accessed January 2019)
[34] Incisive Health & Cancer Research UK. Saving lives, averting costs: An analysis of the financial implications of achieving earlier diagnosis of colorectal, lung and ovarian cancer. Available at: https://www.incisivehealth.com/wp-content/uploads/2018/08/Saving-lives-averting-costs.pdf (accessed January 2019)
[35] Whitlock EP, Lin JS, Liles E et al. Screening for colorectal cancer: A targeted, updated systematic review for the US Preventive Services Task Force. Ann Intern Med 2008;149(9):638-658. PMID: 18838718
[36] Elmunzer BJ, Hayward RA, Schoenfeld PS et al. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS Med 2012;9(12):e1001352. doi: 10.1371/journal.pmed.1001352
[37] Halloran SP. Bowel cancer screening. Surgery 2009;27(9):397–400. doi: 10.1016/j.mpsur.2009.08.013
[38] van Rossum LG, van Rijn AF, Laheij RJ et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008;135(1):82–90. doi: 10.1053/j.gastro.2008.03.040
[39] Benson VS, Patnick J, Davies AK et al. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer 2008;122(6):1357–1367. doi: 10.1002/ijc.23273
[40] Royal Exeter and Devon NHS Trust. Bowel screening service. 2012. Available at: http://www.rdehospital.nhs.uk/patients/services/bowel_cancer_screening.html (accessed January 2019)
[41] Levi Z, Rozen P, Hazazi R et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med 2007;146(4):244–255. PMID: 17310048
[42] Moss S, Mathews C, Day TJ et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut 2017;66(9):1631–1644. doi: 10.1136/gutjnl-2015-310691
[43] Public Health England. Bowel screening to start at 50. 2018. Available at: https://www.gov.uk/government/news/bowel-screening-to-start-at-50 (accessed January 2019)
[44] Public Health England. Health matters: improving the prevention and diagnosis of bowel cancer. 2016. Available at: https://www.gov.uk/government/publications/health-matters-preventing-bowel-cancer/health-matters-improving-the-prevention-and-detection-of-bowel-cancer (accessed January 2019)
[45] National Institute for Health and Care Excellence. Community pharmacies: promoting health and wellbeing. NICE guideline [NG102]. 2018. Available at: https://www.nice.org.uk/guidance/ng102 (accessed January 2019)