A recent Department of Health report identified compliance aids as a contributor to the £150m of avoidable medicines related waste each year in the UK.1 The usefulness of compliance aids has been questioned and the evidence to support their use is poor.2
At North West London Hospitals Trust (NWLHT), in common with a number of other hospitals, we have struggled to address the fact that many health and social care staff recommend (and even insist on) the use of compliance aids for patients who have problems with taking medicines at home.
We use the term “multi-compartment compliance aid” (MCA) to describe medicines storage devices that are divided into days of the week with several compartments per day (eg, morning, lunch, evening and bedtime). Recognising that the MCA issue is an “elephant in the room” — that is, a problem no one wants to acknowledge and the one that is too big to address — we decided to tackle one element only, as an initial effort.
MCAs can aid medicines management for some patients because they act as a visual reminder prompting the taking of medicines. However many health and social care staff perceive MCAs as a solution for all patients, including those who are either non-compliant or confused. Many staff believe that MCA provision will promote safe discharge and help avoid medicines related readmission. As pharmacists we are constantly trying to explain, with the support of the Nursing and Midwifery Council and previous Royal Pharmaceutical Society guidance, that giving a patient an MCA inappropriately could lead to overdose or treatment failure as well as increasing the risk of dispensing errors through secondary dispensing. In addition community pharmacies do not receive any extra payment for dispensing into an MCA or delivering it to the patient’s home.
Some of the problems we are aware of include:
- MCAs can only be used to store some oral solid medicines, usually to a maximum of four doses per day
- Medicines sensitive to light, moisture and temperature may not be suitable for storage in MCAs
- Non-oral and non-solid medicines, cytotoxics, medicines with special handling requirements and “when required”medicines cannot be included in an MCA
- Once in an MCA, individual medicines cannot be easily identified (this affects patients’ choice to take their medicines and matching administration precautions for medicines [eg, take with food])
- MCAs are only suitable for patients oriented in time and place, and who have appropriate manual dexterity
- If four weeks’ worth of medicines are dispensed in an MCA, any medication changes during the four weeks will lead to waste and increased cost
- Some MCAs are not tamper-resistant or child-proof
PANEL1: IS AN MCA APPROPRIATE? KEY FACTORS*
Group A The patient is physically and cognitively able to manage medicines but has requested a multi-compartment compliance aid (MCA). The patient should discuss medicines management support options with their community pharmacy and GP.
Group B The patient has a physical impairment (eg, poor eyesight, limited dexterity) and has formal or informal carers. The MCA is requested because he or she is unable to take medicines out of their original packs. Formal carers can assist by giving the medicine to the patient so original packs can still be used. Discuss options for medication support with informal carers (eg, relatives), including alternatives to MCAs (see Panel 2).
Group C† The patient has a physical impairment but no formal or informal carers. The aid is requested because he or she is unable to take medicines out of their original packs. Assess the patient’s ability to take medicines out of non Click-Loc containers or an MCA. (Note that the type of MCA issued by the hospital may differ to that provided by the community pharmacy). If he or she is unable to manage either of the two options, discuss medication support options with the medical and social care team.
Group D† The patient has a cognitive impairment and has formal or informal carers. The MCA is requested because he or she has poor memory or poor orientation in time and place. Discuss medication support with informal carers. If formal carers are supporting medication they may require an MCA. In this case the hospital pharmacy can supply one but only as a sealed disposable box. Ensure social care calls (eg, breakfast, lunch and dinner) coincide with timing of medication.
Group E The patient has a cognitive impairment but no formal or informal carers. The MCA is requested because he or she has poor memory or poor orientation in time and place. If the patient cannot safely self medicate discuss options for support with the medical team, social team, GP and community pharmacist before discharge.
† If the patient falls into group C or D, assess the following medicine factors before issuing an MCA:
- If the medicines require more than four doses per day an MCA is not appropriate. Consider alternative medicine support. GP to review post discharge.
- If the regimen is unstable (eg, frequent changes), an MCA is not appropriate. Consider alternative medicines support. GP to review post discharge.
- If the regimen only involves regular oral solid medicines an MCA is suitable and can be supplied.
- If medication does not coincide with the timing of care calls, the pharmacist and medical team should review the regimen. For example, can the timing of the medication be altered without reducing efficacy? If not, then an MCA is not appropriate and alternative medicine support should be considered.
- If the regimen includes medicines that cannot be placed into an MCA (eg, for stability reasons) as well as regular oral solid medicines, contact the carer, care agency or social services before discharge, to ensure they are able to administer medicines that are not dispensed in an MCA.
*Adapted from North West London Hospitals NHS Trust policy
Policy
Assessing a patient’s medicines support requirements is essential in promoting compliance. Although we recognise that this should, ideally, be undertaken with the patient in a domiciliary setting rather than when the patient is acutely unwell in hospital, we believe that hospital admission is an opportunity to identify medicines related issues, including those involving MCAs. We, therefore, developed an MCA policy to address new requests for MCAs, working with key stakeholders from nursing, medical, therapy and social care backgrounds within the trust and taking into account views from primary care health and social care stakeholders, including care agencies. Although it was not possible to satisfy all the needs of the stakeholders, we were able to reach a compromise and create a policy that they could all agree on.
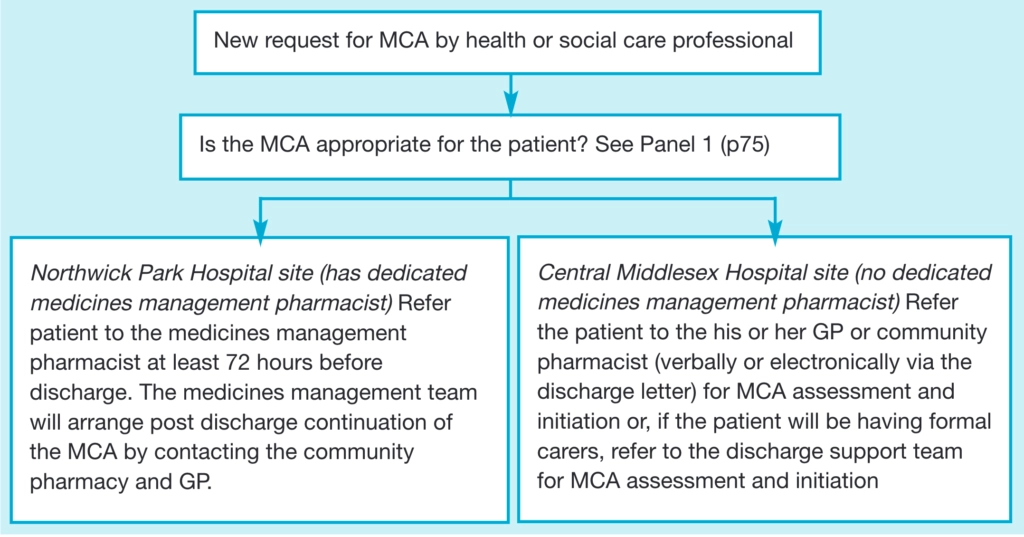
The policy mandates liaison between the patient, pharmacist, nursing staff and medical staff in hospital with agreement from the patient’s GP and the community pharmacy to be obtained before supply of an MCA. Information regarding the process of supplying an MCA to a patient is provided to all healthcare professionals. The process is outlined in the flow diagram above and Panel 1 describes key factors that will indicate if an MCA is appropriate. Patients meeting set criteria are referred to the medicines management team which will carry out an adherence assessment and organise the post discharge support. The assessment details are documented in the patient’s notes. The policy has been ratified by the trust’s Drugs and Therapeutics Committee and is available on the trust intranet.
PANEL 2: EXAMPLES OF ALTERNATIVES TO AN MCA
- Simplify regimen
- Use a reminder chart
- Supply a list of medicines in the appropriate language
- Supply a large font information sheet
- Supply medicines in non click-lock containers
- Ask carer or relative to administer medicines
Dispensing and documentation
We have created a standard operating procedure for pharmacy staff, which details the process of screening, dispensing and checking an MCA. Pharmacy staff dispense medicines into individual cartons that are then checked by a pharmacist or accredited checking technician, who fills the MCA. With the help of the RPS, which is currently creating guidance around stability of medicines in MCAs [see Panel 3], we were able to create a checklist of characteristics of medicines that can be considered for MCA dispensing. We chose not to use currently published reference guides because they do not account for the variety of generic products we use. Instead we refer to summaries of product characteristics for branded products and the checklist provided by the RPS for generic products, as well as the storage instructions on the outer packaging of the medicine. In order to protect the medicine within the MCA, each MCA has an additional cautionary label stating that the MCA should be protected from light, stored at room temperature and in a dry environment.
Where patients were using an MCA before admission, we document the discharge information during the medicines reconciliation process in the pharmacy section of the drug chart. We also affix an orange sticker which states: “This patient is on a medication compliance aid. Please allow plenty of time to arrange for discharge”.
We supply one week’s worth of medicines in a sealed disposable MCA (Nomad blister pack), but we will supply a reusable MCA on the patient’s request. Using a secure fax procedure and a standard template, we fax the medicines section of the electronic discharge letter to both the GP and community pharmacist. This helps the community pharmacist to reactivate the MCA process post discharge and gives a week for the process to be reinstated.
All pharmacy staff have been trained and we have a rolling programme to train nursing staff, junior doctors, therapists and social care staff. The policy was launched this month.
Measuring success
Our current dispensing workload includes around 50 MCAs a month. Before the initiation of the policy we accepted all MCA requests, regardless of whether these requests were appropriate or not. As a result of the policy, all new requests for an MCA will be assessed by the medicines management team so inappropriate initiation of MCAs should be avoided. We will audit the service in six months and review the number of MCA requests accepted and rejected and examine reasons for rejection.
In future we would like to work with our community pharmacy colleagues to enable referral of patients at risk of preventable medicines related issues for targeted medicines use reviews. Although we recognise that our policy will only address as small part of the MCA issue, we hope that we have started to tackle the elephant in the room.
PANEL 3: RPS GUIDANCE
Although the use of monitored dosage systems (MDS) and compliance aids is widespread in pharmacy practice, these types of packaging systems should only be supplied if an individual assessment of a patient suggests they will provide benefits to the patient. They should not be supplied simply for the convenience of patients or their carers.
The RPS is currently developing guidance for members on the use of MDS and compliance aids. The guidance is expected to be published this year and will be available from www.rpharms.com. The aim of this guidance is to assist members in making professional judgements about whether MDS or compliance aid packaging is suitable for a particular patient and in assessing which medicines may be included in these systems.It will consider possible liability issues if MDS or compliance aids are supplied, the potential risks to patients of using this form of packaging, and the implications of theDisability Discrimination Act. In addition, it will advise on the types of dosage forms that maybe unsuitable for inclusion in such systems and will provide members with information that allows them to use their pharmaceutical science knowledge to assess the suitability of an individual medicinal product for inclusion in MDS or compliance aids. Members will also be signposted to relevant sources of additional information. — Colin Cable, pharmaceutical science information adviser at the Royal Pharmaceutical Society
References
- Evaluation of the scale, causes and costs of waste medicines, York Health Economics Consortium/School of Pharmacy, University of London, November 2010.
- Oboh L. FAQs on the use of multicompartment compliance aids. Lambeth NHS Trust, June2009.


