This content was published in 2009. We do not recommend that you make any clinical decisions based on this information without first ensuring you have checked the latest guidance.
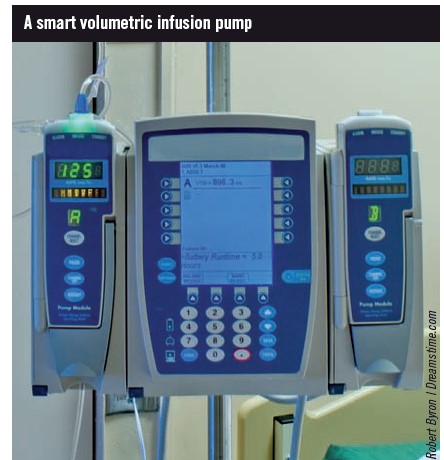
An appropriate intravenous medicine has been prescribed. It has been supplied correctly to the ward. Yet, a member of ward staff has entered the wrong rate into the infusion pump and an administration error is about to occur. Except . . . the pump — a “smart” device — has recognised the mistake and will not proceed with the infusion.
This is not a work of science fiction: medication error reduction software (MERS) now comes as standard in most intravenous infusion pumps, providing a new type of safety check in IV drug administration. Risk reduction is an NHS priority; therefore the place of MERS in the modern NHS requires exploration (see Box 1).
At Southampton University Hospitals NHS Trust (SUHT), we have attempted to produce a drug library to allow implementation of MERS indifferent clinical areas simultaneously to explore feasibility for a trust-wide roll-out. Implementation of this library in two different smart infusion devices is under way.
Drug library development
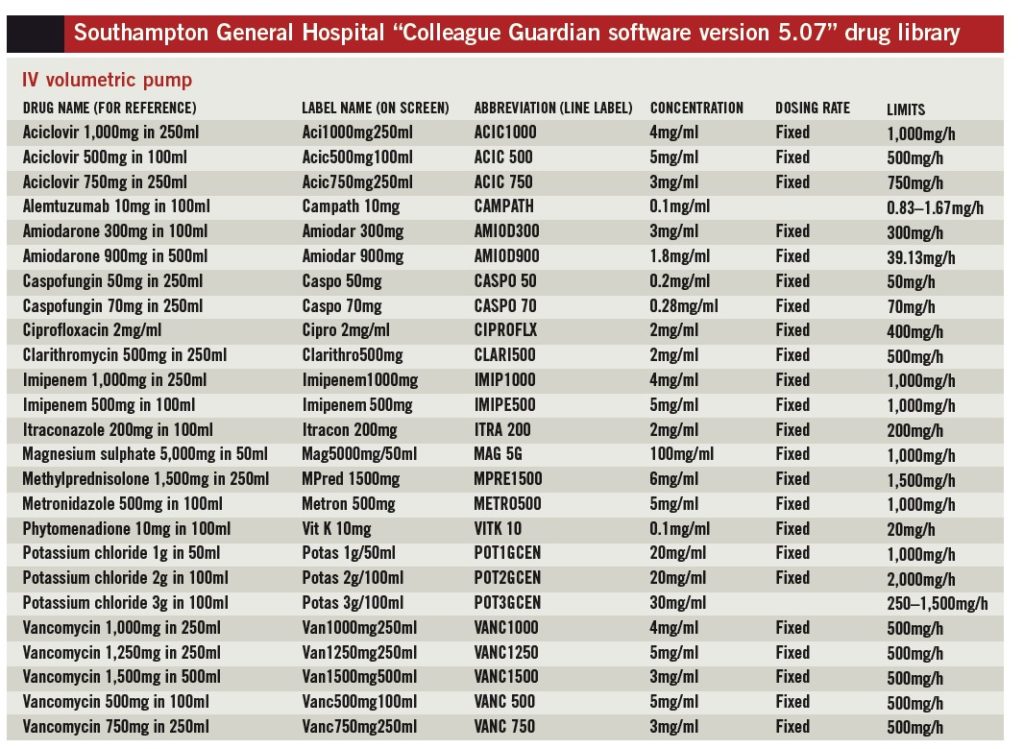
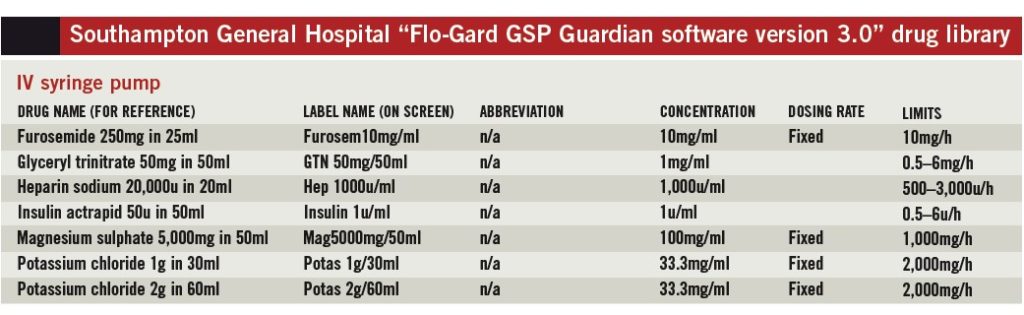
SUHT uses the Baxter Flo-Gard GSP IV syringe pump and the Baxter Colleague IV volumetric pump; each uses Guardian MERS. Central to MERS development is the creation of a drug library with rationalised infusion rates and concentrations across several ward areas [8]. It is important to have a clear idea of how the pumps function, and what is to be achieved with the pumps, before developing the drug library.
Such planning can help ensure the correct library parameters are set. We, the team at SUHT, chose four different ward areas for introduction of MERS, selected to allow for comparison between high and low volume usage given the different patterns of error seen in each area:
- Two ward areas using IV syringe pumps: an intensive care unit with high-volume usage and a standard cardiology ward with less frequent usage
- Two ward areas using IV volumetric pumps: a high dependency unit and an oncology ward
Initially we identified key players and governing groups at SUHT to take part in the library development. Involving parties in the order listed in Box 2 made for a lengthy process; however, we believe it was necessary to ensure smooth implementation.
Multidisciplinary working
Four multidisciplinary (MD) meetings were convened to compile the drug library entries for the four clinical areas. Each MD group reviewed the following documentation relevant to their clinical area before deciding on a list of drugs to include:
- Recent adverse event and error reports concerning the administration of IV infusions
- Pharmacy reports for the most frequently used IV medicines
- Up-to-date lists of medicines stocked in the ward
Also examined were samples of drug libraries from other trusts, as well as a risk assessment report for 100 commonly used injections in adults (from the joint NHS pharmacy technical services groups).
“Smart” infusion pumps have the potential to reduce intravenous drug administration errors. But these devices are only as reliable as the infusion information they contain — and the person operating them.
Rationalisation
For each drug included in the library, a fixed infusion concentration needed to be agreed and soft and hard dosing limits stated (see “Dosing limits”). This is a major undertaking because several prescribing teams practise within certain specialist areas; accordingly, rationalisation has to occur within each clinical specialism before it can even be attempted between specialisms. Some areas were simpler than others, as illustrated by the approximate numbers of teams listed here:
- Intensive care — seven surgical teams
- Cardiology — eight cardiology teams
- High dependency — 25 surgical teams
- Oncology — one team
The need to specify a fixed concentration excludes many medicines from the library since the MERS used at SUHT determines infusion rates in terms of drug quantity per minute (e.g., mg/min, μg/min, units/min).

Millilitres per hour is not available as a dosing mode in our MERS version; consequently, drug doses individualised to patients based on weight or surface area but infused in a fixed volume over a fixed time cannot be programmed. Medicines excluded for this reason are often high-risk, for example chemotherapy. This poses some challenges for the future, among them: investment in smart infusion device technology upgrades; dose-banding to allow a choice of infusion strengths; and rationalisation of protocols between hospitals.
Box 1: Background
National Patient Safety Agency research indicates that errors involving injectable medicines are higher than for other dosage routes. Some 24% of medication incidents reported to the National Reporting and Learning Service relate to injectable medicines [1] and 58% of incidents that lead to death or severe harm are associated with parenteral administration.
In one UK study, one or more errors occurred in at least 49% of intravenous medicine doses; administration errors accounted for 30% of these [2]. Other work conducted in the US has shown the most commonly identified reason for administering the wrong dose was an error in programming the IV infusion pump [3]. The NPSA recommends double-checking systems — medication error reduction software (MERS) in smart IV infusion pumps and syringe drivers is one such system.
A variety of data has been published on the use of smart IV infusion technology showing between no measurable impact on serious medication error rate [4] and possible prevention of 10-fold overdoses. [5]. Authors are in agreement that so-called “smart pumps” are useful tools in designing risk out of the system. This technology has been available for some time. In the US and Canada dose-checking software has been used for many years. The US Institute for Healthcare Improvement core processes for administering medicines includes a recommendation that smart-pump technology be used to reduce IV administration errors.
Interest in smart IV infusion technology has been shown at Southampton University Hospitals NHS Trust (SUHT) not only for risk reduction but also for audit purposes. With MERS enabled, data downloads from the smart pumps can flag up potential errors prevented and therefore contribute to reporting and training. One of the barriers to the implementation of smart pumps across an acute hospital like SUHT is the lack of rationalisation in drug concentrations. [6] This is seen throughout UK NHS practice, where few infusions are made centrally in a pharmacy — the majority being prepared by nursing staff on the ward [7, 8].
Rationalisation of drug concentrations for injection is included within the action points of NPSA alert 20. Current smart pump technology relies on fixed infusion concentrations and may be a useful tool in enforcing the rationalisation of high-risk IV drug concentrations.
Dosing limits
Soft dosing limits are those infusion rates within which an infusion can be safely administered — if an IV infusion is programmed outside these soft limits the pump will alarm to alert the user but on confirmation will allow an override. Hard limits are those outside which the pump will block administration of an infusion— this cannot be overridden. We chose to use soft limits only, because this implementation of MERS was a pilot exercise and we did not want to prevent overrides completely in case specific parameters had been overlooked for particular infusions used at SUHT.
Moreover, in emergencies or unusual cases we wanted the operator to be able to access higher or lower doses (e.g., for a patient who is obese and highly insulin-resistant, requiring a massive dose of insulin; or for an anorexic patient, sensitive to heparin, who requires a tiny dose). The MD groups chose for inclusion in the library a limited selection of medicines for which they felt the risks to patients from moving outside the recommended infusion rates were high.
Minimising selection errors
An on-screen label with a 14-character limit also needed to be specified for each medicine. The labels occupied much of the MD groups’ discussion time because the choice of text describing drug and concentration is crucial to minimise selection errors. The risk management pharmacist was a key adviser for this part of the process. Selection error potential emphasises a weakness in the use of smart pumps since these rely completely on the user selecting the correct drug and concentration when programming an infusion.
Creation of tailored lists of drugs called “personalities” within a drug library (rather like playlists on an MP3 player) helps reduce selection errors— the core library of all approved drugs is loaded into the pump but different personalities only allow the user to select those medicines relevant to his or her clinical area. Nonetheless, the user still needs to select the correct personality. We developed two personalities for the MERS within the IV volumetric pump (one for each clinical area involved).
Box 2: Key players
- Parenteral therapy group — formed to implement an action plan in response to National Patient Safety Agency alert 20
- Risk and patient safety group — strategic and operational role in implementation of risk reduction and patient safety initiatives across the trust
- Medical equipment library and biomedical engineering — to give a realistic view of the resources required and to help deal with the practicalities of implementation
- Specialist nurse within each clinical area — to co-ordinate training of staff using the drug library
- Lead clinician (or clinician nominated by lead), specialist pharmacist and lead/specialist nurse foreach clinical area — to design drug library
- Risk management pharmacist — to review and check proposed drug library for accuracy
- Divisional clinical governance groups appropriate for each clinical area — to approve the use of medication error reduction software and the proposed drug library within their clinical area
- Trust drugs committee — to approve the use of the drug library and its parameters, doses, etc, within the trust
Consensus
After drafting drug lists for each of the four trial clinical areas, the two lists of drugs for use in the IV syringe pump were compared, as were the two lists for the IV volumetric pump. Where two clinical areas had a conflicting drug infusion (e.g., potassium chloride 1g/100ml versus potassium chloride 1g/50ml, or potassium chloride 1g/50ml over 1h versus potassium chloride 1g/50ml over 30min) that item was discussed further by the MD groups.
If a consensus could not be reached for an individual infusion, the drug in question was removed from the final list. Thus we established a core drug library for the syringe pump and a core library for the volumetric pump. The formation of standardised libraries in this way was appropriate because, like many NHS hospitals, SUHT uses a medical equipment repository to share pumps between clinical areas.
Allowing multiple concentrations for single medicines would occupy many fields in the MERS and limit the inclusion of additional items if the library was to be developed for the whole hospital at a later date. Infusions put forward for the MERS drug library but not included because of conflict between clinical areas were: magnesium sulphate 2g/25ml (syringe pump, intensive care); potassium phosphate 10mmol/100ml (volumetric pump, high dependency); and potassium phosphate 10mmol/500ml (volumetric pump, oncology).
These infusions remain unmonitored by MERS at present but, because of their high-risk nature, this will be reviewed for future development.
A smart move?
Developing a MERS drug library for use in more than one clinical area is certainly a challenge (our four clinical areas involved over 40 prescribing teams). If this technology is to be employed —particularly where infusion devices are shared from a medical equipment store — rationalisation of IV infusion administration across different clinical areas is essential.
The UK literature, to date, reports on the use of smart infusion drug libraries in single specific clinical ward areas where rationalisation is more easily achieved [9]. If a clinical area holds its own stock of pumps and these are not used in other areas, developing a library specific to that area appears the best option.
Perhaps a first step to broadening the use of MERS and rationalising infusion practice across more than one ward area could be to focus on rationalising one or two specific high-risk medicines to be administered using MERS across a hospital (e.g., IV potassium) [7]. To inform future library development it might also be useful to collect data across an entire acute trust — exploring variation in the delivery of similar infusions and targeting service development accordingly.
Resources required to produce and implement a drug library should not be underestimated. Significant time commitment from many individuals is required. The time span through MD group set-up, risk management, governance groups, prescribing committee approvals, staff training, library validation and data uploading was six to nine months.
To co-ordinate this alone took around three hours of pharmacist input per week. Maintenance of the library should also not be overlooked.
Nevertheless, if serious errors will be avoided and patient safety improved, development of a drug library for use in smart IV infusion devices is definitely a smart move.
References
- National Patient Safety Agency. Patient safety alert 20: Promoting safer use of injectable medicines. March 2007.www.npsa.nhs.uk (accessed 23 April 2009).
2. Taxis K, Barber N. Ethnographic study of incidence and severity of intravenous drug errors. BMJ 2003: 326:684–8.
3. Adachi W, Lodolce AE. Use of failure mode and effects analysis in improving the safety of IV drug administration. American Journal of Health-System Pharmacy 2005; 62:917–20.
4. Rothschild JM, Keohane CA, Cook EF, et al.A controlled trial of smart infusion pumps to improve medication safety in critically ill patients. Critical Care Medicine 2005; 33:533–40.
5. Fortescue EB, Kaushal R, Landrigan CP, et al. Prioritising strategies for preventing medication errors and adverse drug events in paediatric inpatients. Paediatrics 2003; 111:722–9.
6. San Diego Patient Safety Consortium. Getting started kit: Safe administration of high-risk IV medications. 2006.www.hasdic.org/documents/tool-kit-high-risk.pdf (accessed 23 April 2009).
7. Hardy L, Mellor L. Risk assessment of parenteral product preparation across secondary care acute trusts in the north of England. Hospital Pharmacist 2007;14:58–64.
8. Borthwick M, Woods J, Keeling S, et al. A survey to inform standardisation of intravenous medication concentrations in critical care. Journal of the Intensive Care Society 2007;8:92–6.
9. Murdoch L. Safety features of smart infusion. Hospital Pharmacy Europe 2007; (35):65.