DRLIWA/ISTOCKPHOTO.COM
This content was published in 2008. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
During a hospital stay, most patients undergo at least one invasive procedure that carries a risk of infection. Such procedures include the insertion of intravenous devices, urinary catheterisation and surgery. As a member of the healthcare team, pharmacists must remain aware of the risks of infection, and provide proactive advice on how to treat or reduce these risks.
Catheter-associated UTI
Urinary tract infections (UTIs) are one of the most predominant types of hospitalacquired infection — the majority of which occur after the insertion of a urinary catheter. The prevalence of such UTIs has led to the publication of national guidelines for preventing infection associated with the short-term use of indwelling urethral catheters (IUCs), the type of urinary catheter that is most commonly associated with causing UTIs.1
Cause of infection
The insertion of a urinary catheter allows the entry of bacteria into the urinary tract. The route of entry can be:
- Intra-luminal — urine in the drainage bag becomes contaminated, then flows back through the IUC into the bladder
- Extra-luminal — bacteria from the hands of healthcare workers or the skin around the entrance to the urethra travel along the outside of the IUC
Bacteriuria (the presence of bacteria in the urine) is a common consequence of urethral catheterisation. After insertion of the device, 3–6 per cent of patients develop bacteriuria per day, however this risk accumulates.2 Therefore, about half of the patients who are catheterised for 10 days will become bacteriuric. This is normally asymptomatic and resolves after removal of the catheter, however 20–30 per cent of those patients will develop a catheterassociated UTI, which often extends the hospital stay.
Escherichia coli is the most common infective organism, but Proteus spp, Enterobacter spp, Pseudomonas aeruginosa, Candida spp and meticillin-resistant Staphylococcus aureus (MRSA) can also cause UTIs.
Complications
The infecting bacteria can form a biofilm that adheres to the lining of the IUC or drainage bag — giving these bacteria a survival advantage. Many antibacterial drugs exhibit poor penetration into biofilms, which renders these drugs ineffective.
In addition, 1–4 per cent of patients with catheter-associated UTIs develop bacteraemia (bacterial infection in the bloodstream), which is fatal in 13 per cent of cases.3
Diagnosis
A fresh urine sample should be taken from all catheterised patients for whom a UTI is suspected, and sent to the microbiology department for culturing. Dipstick testing for nitrites and leukocytes is not advised, because urine from these patients is usually colonised with bacteria, regardless of whether a UTI has developed. Therefore such testing leads to a high rate of false-positive results.
Treatment
Antibacterial treatment should be delayed, if possible, until a positive microbiological result has been obtained. This avoids indiscriminate antibacterial use in patients that do not have a UTI. It also allows the antibacterial sensitivity of the infecting organism to be determined. A seven-day course of oral antibacterials is usually sufficient to treat the infection, however any patient who is bacteraemic should receive prompt intravenous antibacterial treatment.
Reducing the incidence
In order to reduce the incidence of catheter-associated UTIs, urinary catheters should only be used when other measures (eg, intermittent catheterisation, use of incontinence pads) are inappropriate. Also, the need for the catheter should be reviewed regularly so that it can be removed as early as possible.
The risk of developing an infection depends on the catheterisation process, the standard of care while the catheter is in place and the patient’s susceptibility to infection. The following measures reduce this risk:
- Using the correct, aseptic technique to insert the catheter
- Positioning the drainage bag below the level of the bladder to prevent backflow of urine
- Ensuring appropriate hand hygiene and using gloves while handling all sterile catheterisation equipment
- Emptying the drainage bag only when it is full
Suprapubic and condom catheters may cause a lower incidence of UTIs than urethral catheters,2 therefore can be considered where appropriate. There is also some evidence that catheters coated or impregnated with silver alloy are associated with a lower incidence of UTIs,4 but further research is needed.
Prophylactic antibacterials can be used before or during catheterisation, however this has only proved beneficial in small subgroups of patients (eg, men undergoing a transurethral resection of the prostate).
IV device infection
Infections can result from the insertion of any IV administration device, whether it feeds into a major central vein (eg, subclavian vein central lines and peripherally inserted central venous catheter [PICC] lines) or a peripheral vein (eg, Venflons).
Causes of infection
IV device infections are usually caused by micro-organisms from the following sources:
- The hub of the catheter — transferred from the skin of healthcare workers when handling the device
- The skin around the site of insertion
- The administered fluid (although such contamination is rare)
The risk of infection is affected by the process of insertion and the standard of care while the catheter is in place. Contamination with micro-organisms can occur whenever the device is handled (eg, to administer an IV drug) or when the dressings around the insertion site are changed, which can lead to infection.
Infection is normally caused by organisms that colonise the patient’s skin. Of these commensal bacteria, S aureus is the most common and tends to be the most pathogenic.
Complications
Infecting organisms can travel along the device into the bloodstream to cause bacteraemia. A third of all nosocomial bacteraemias can be attributed to central venous catheter infections,5 so national guidelines for preventing such infections have been produced.1
Alternatively, infecting organisms can migrate into the tissue surrounding the insertion point to cause local infections (eg, cellulitis).
Treatment of bacteraemia
The treatment of bacteraemia depends on the suspected infecting organism and the severity of illness. Where possible, the device that caused the infection should be removed. This is not usually a problem, unless uninterrupted IV access is required (eg, for haemodialysis). For infection with some low-virulence organisms (eg, Staphylococcus epidermidis), removing the device can be sufficient to resolve the infection.
In most cases, prompt treatment with IV antibacterial drugs is required. The duration of treatment depends on the susceptibility of the infecting organism, whether or not the catheter is removed and patient-specific factors (eg, allergies to first-line antibacterials, immunosuppression). Specialist advice from microbiology or infectious disease specialists should be sought in all cases.
Treatment of cellulitis
Similarly to bacteraemia, cellulitis is treated by removing the cannula and starting antibacterial treatment. Mild cellulitis can be treated orally, although severe cellulitis requires initial IV treatment.
First-line treatment is flucloxacillin. For patients who are penicillin-allergic, oral clindamycin or IV vancomycin are suitable. Improvement is generally seen within 24–48 hours. Treatment for seven to 14 days is normally required, depending on the patient’s response.
If the patient is colonised with MRSA or has not responded to initial treatment, MRSA-active agents (eg, vancomycin) may be required.
Reducing the incidence
Devices should only be inserted when ongoing IV access is needed and should be removed as early as possible — often within a few days. Some tunnelled central line devices (eg, Hickman lines) can be left in place for several months.
The site of insertion should be inspected daily for signs of infection (eg, redness, swelling or pain). The device must be removed immediately if any of these signs is present, to prevent a local infection progressing to bacteraemia.
The impregnation of temporary IV devices with antimicrobials or antiseptics has shown to reduce the incidence of bacteraemias.6
Surgical site infection
Surgical site infections (SSIs) increase the financial burden on hospitals in terms of additional drug expenditure and longer hospital stay. However, such infections often present after the patient has been discharged, so can be difficult to diagnose and treat promptly. Swift treatment is essential because infections are potentially fatal.
Cause of infection
SSIs are normally caused by bacteria that are introduced to the site of surgery during the procedure. Alternatively, in the case of non-elective or urgent surgery (eg, to repair a wound), the micro-organisms may be present before surgery.
Risk factors for developing an SSI include:
- Insertion of prosthetic implants
- Long procedures
- Severe co-morbidities
Complications
If an infected site contains prosthetic material, the removal of the material is normally required before the infection can be treated. If the material cannot be removed, a long-term course of antibacterial treatment may be necessary.
Preventing infection
Antibacterials are often administered prophylactically to prevent an SSI if:
- The likelihood of infection is high (eg, surgery on an open wound that is more than four hours old)
- The consequence of infection is severe (eg, surgery involving the brain or removal of prosthesis)
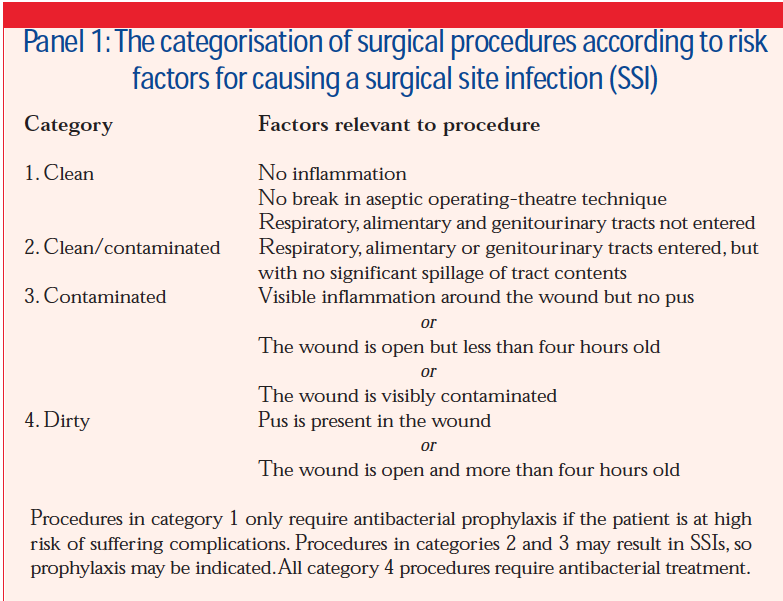
Guidelines have been produced by the Scottish Intercollegiate Guidelines Network to categorise surgical procedures in terms of infection risk. This helps to determine whether the patient should receive antibacterial prophylaxis during the procedure. The categories are outlined in Panel 1.
The antibacterial used must be active against the organisms that commonly cause SSIs. However the potential benefit of antibacterial prophylaxis must be balanced against the risks (eg, Clostridium difficile-associated diarrhoea).
The timing of prophylactic antibacterials is crucial. IV doses should be administered immediately before a procedure, whereas oral doses should be given an hour before. High blood concentrations of antibacterials must be maintained throughout the procedure, therefore some short-acting drugs (eg, penicillins and cephalosporins) will require repeat doses if the procedure lasts longer than four hours.
Continuing prophylactic treatment postoperatively is not usually necessary, unless an infection is presumed or deemed extremely likely (eg, after emergency surgery on a dirty wound).
Treatment
The strategy used to treat an SSI depends on the type of surgery and severity of infection, although initial antibacterial treatment is usually empirical. Such treatment should be reviewed regularly, because it is based on an assumption of the infecting organism.
The duration of treatment depends on the severity of infection and the response to treatment. If an infection involves an uncomplicated area (eg, a superficial skin wound) with no prosthetic material and improves rapidly, it may only require a seven to 10 day course of antibacterials. Such infections can usually be treated orally, or switched from IV to oral treatment within a few days.
If an infection does not improve with initial antibacterial treatment, it may require further surgical intervention (eg, debridement [removal of necrotic or infected material]). Tissue or fluid samples should be sent for bacterial culturing to identify the infecting organism and allow targeted antibacterial therapy.
Some patients (eg, those suffering from bone infections) may require long courses of antibacterials (IV followed by oral). To reduce the length of admission and the risk of further infection, some patients are allowed to complete the course of IV antibacterials at home. Home IV therapy is discussed on p16.
References
- Dancer SJ (ed). Epic2. National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. The Journal of Hospital Infection 2007;54:S1–S64.
- Saint S, Lipsky BA. Preventing catheter-related bacteriuria: Should we? Can we? How? Archives of Internal Medicine 1999;159:801–8.
- Bryan CS, Reynolds KL. Hospital-acquired bacteremic urinary tract infection: epidemiology and outcome. The Journal of Urology 1984;132:494–8.
- Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infection: a meta-analysis. The American Journal of Medicine 1998;105:236–41.
- Coello R, Charlett A, Ward V, Wilson J, Pearson A, Sedwick J, et al. Device-related sources of bacteraemia in English hospitals — opportunities for the prevention of hospital-acquired bacteraemia. The Journal of Hospital Infection 2003;53:46–57.
- Sampath LA, Tambe SM, Modak SM. In-vitro and invivo efficacy of catheters impregnated with antiseptics or antibiotics: Evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infection Control and Hospital Epidemiology 2001;22:640–6.
