Carol & Mike Werner / Science Photo Library
Insulin is a hormone produced by the beta cells in the islets of Langerhans of the pancreas. It allows the body to use glucose from food to provide energy and to store glucose for future use. Blood glucose levels are controlled by a feedback system that has multiple inputs from other hormonal controls; however, insulin is the only hormone that can lower blood glucose levels. For more information on the feedback system that controls blood glucose levels and the impact of insulin, see ‘Insulin feedback system’.
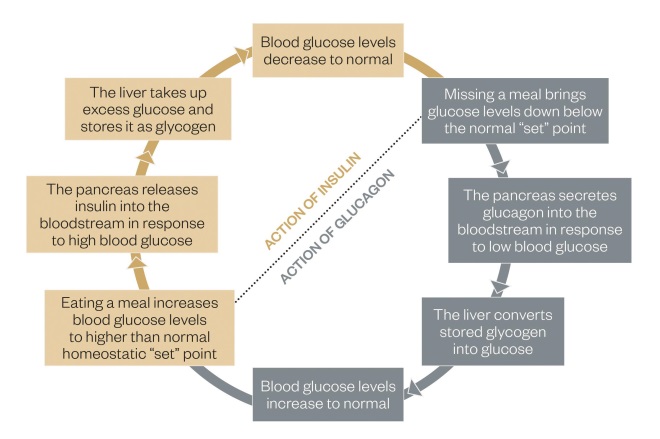
Figure 1: Insulin feedback system
The feedback system outlines how blood glucose levels are controlled and details the action of insulin.
In diabetes mellitus, insulin levels (and the related feedback system) are abnormal. There is a distinct difference between different types of diabetes (e.g. type 1, type 2, gestational, cystic fibrosis-related) and, as a result, insulin therapy must be tailored to the needs of the individual. This article will examine how these decisions are made and how the current range of insulin preparations allows prescribers to individualise each regimen. For a list of the current insulins available in the UK market, see ‘Insulin preparations available in the UK’. Animal insulin is still available; however, these are no longer recommended for initiation in patients and so have been left out of the table.
It should be noted that there is great variability in the absorption and action of insulin between different patients that is caused by multiple factors. The figures in ‘Insulin preparations available in the UK’ provide a guide for action, but each patient should be considered individually.
| Name | Onset (approx.) | Peak activity (approx.) | Duration of action (approx.) | Cost (based on 5x3mL cart) |
|---|---|---|---|---|
| Source: Created using http://www.joslin.org/info/insulin_action.html, MIMS UK Insulin Preparations Table and BNF Sept 2015. | ||||
| Human basal insulin (NPH insulin) | ||||
Humulin I Insulatard Insuman Basal | 90 mins–4 hours | 4–12 hours | 12–20 hours | £19.08 £22.90 £17.50 |
| Human short-acting insulin | ||||
Actrapid Humulin S Insuman Rapid | 30–60 mins | 2–5 hours | Up to 12 hours | £7.48 (10mL vial) £19.08 £17.50 |
| Human Mix insulin (Biphasic) | ||||
Insuman Mix 15 Insuman Mix 25 Insuman Mix 50 Humulin M3 | 30 mins–1 hour | 2–4 hours | 12–20 hours | £17.50 £17.50 £17.50 £19.08 |
| Analogue basal insulin | ||||
Lantus Levemir Tresiba U100 and U200 Abasaglar Toujeo U300 | 30 mins–1 hour | – | 24 hours (Tresiba and Toujeo have extended action up to 36–42 hours) | £41.50 £42.00 £72.00 £35.28 £33.13 |
| Analogue short-acting insulin | ||||
Humalog U100 and U200 Apidra Novorapid | 10–30 mins | 1–3 hours | 2–5 hours | £28.31 £28.30 £28.31 |
| Analogue mix insulin (Biphasic) | ||||
Novomix 30 Humalog Mix 25 Humalog Mix 50 | 10–20 mins | 1–4 hours | 24 hours | £28.79 £29.46 £29.46 |
Type 1 diabetes
A complete lack of endogenous insulin characterises type 1 diabetes. As a result, insulin therapy in these patients is designed to mimic physiological levels as closely as possible. When considering physiological insulin levels, although there is a large amount of insulin released in the presence of food, there is also a constant background level of insulin release, including overnight during the fasting period. This is because glucose is constantly required and therefore constantly released by the liver. Up to 50% of insulin produced by the body in 24 hours is released in these ‘fasting’ periods and is referred to as the basal or background level.
On account of a lack of endogenous insulin production, a patient with type 1 diabetes is usually maintained on a ‘basal-bolus’ regimen consisting of a long-acting analogue insulin to replicate the basal level and meal time bolus doses of short-acting analogues; see ‘Intensive insulin replacement compared with natural, non-diabetic insulin secretion’ for further information on action. Analogues are favoured as they most closely mimic normal insulin release (i.e. long-acting analogues have little or no peak and short-acting analogues have a fast onset of action that fades away quickly, see ‘Insulin preparations available in the UK’).
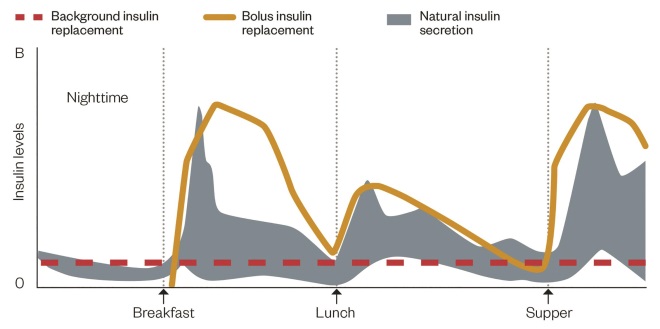
Figure 2: Intensive insulin replacement compared with natural, non-diabetic insulin secretion
Source: www.dtc.ucsf.edu. Available by written permission of The Regents of the University of California
Due to a lack of endogenous insulin, patients with type 1 diabetes are usually maintained on a ‘basal-bolus’ regimen consisting of a long-acting analogue insulin to replicate the basal level and meal time bolus doses of short-acting analogues. Analogues are favoured as they most closely mimic normal insulin release.
The National Institute for Health and Care Excellence (NICE) guidelines published in August 2015 recommend “the multiple basal-bolus regimen as the regimen of choice in all patients with type 1 diabetes” and advise that “newly diagnosed patients are not offered the option of non-basal bolus regimens”[1]
. This is because the evidence reviewed showed that early and immediate glycaemic control in patients prevents the development of long-term complications such as retinopathy and nephropathy. Therefore, the practice of starting a patient on one or two injections of basal or mixed insulin to ‘get them used to it’ has fallen from favour and should not be encouraged. A small number of patients may be maintained on BD insulin products because of their personal circumstances. This is an exception; see ‘Patient factors’ for a discussion of factors affecting insulin choice. Insulin initiation in type 1 diabetes should only be undertaken by a healthcare professional specialised in diabetes and specifically in the management of type 1 diabetes. As structured education is deliberately delayed for some time after diagnosis (usually offered 6–12 months after diagnosis), it is essential that insulin initiation is combined with dietary advice from a dietitian specialised in carbohydrate-counting and insulin adjustment.
This regimen requires patient engagement. Four or five injections a day and multiple capillary blood glucose tests are needed, preferably at least four a day (before each meal and at bedtime). Adult patients with type 1 diabetes should also be offered structured education and be able to ‘carbohydrate-count’ or ‘carb-count’. This is a technique where carbohydrate intake is ‘counted’, which allows patients to adjust their dose of insulin to the meal they are about to eat. Examples of such programmes are DAFNE, XPERT, DAFYDD and ASPIRE and availability depends on the locality of the patient[1]
. This allows maximum flexibility in timings and diet, but also means patients are at higher risk of hypoglycaemia and hyperglycaemia. The lack of endogenous insulin production and altered feedback mechanisms mean that miscalculation of doses, lack of engagement or illness can result in diabetic ketoacidosis (DKA) or unconscious hypoglycaemia, which must be avoided if at all possible. However, with suitable education and adjustment, this regimen provides patients with the best opportunity to maintain good glycaemic control, avoid hypoglycaemia and minimise the risk of developing complications. Insulin pumps can also be used to maintain tight glucose control or deal with difficult-to-manage type 1 diabetes; however, discussion of these is beyond the scope of this article.
Type 2 diabetes
Patients with type 2 diabetes have a different disease. Unlike with type 1 diabetes, patients often have endogenous insulin production (although at less than necessary levels) but also have highly increased resistance to insulin action, which results in hyperglycaemia. This is why patients can be maintained on oral therapy alone to aid the use of insulin already produced by their body. The three main regimens are discussed further below and are highlighted in ‘Insulin profiles for common insulin regimens’.
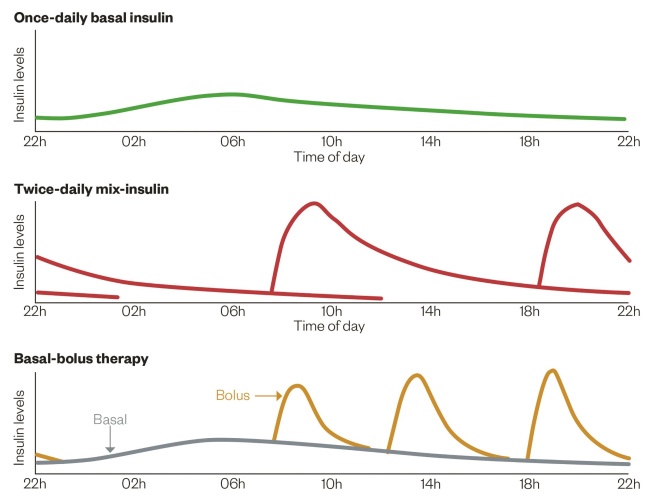
Figure 3: Insulin profiles for common insulin regimens
Source: Diapedia. http://diapedia.org/management/8104117123/insulin-regimens
Unlike patients with type 1 diabetes, those with type 2 diabetes often have endogenous insulin production (although at less than necessary levels) but also have highly increased resistance to insulin action, which results in hyperglycaemia. Patients can be maintained on oral therapy alone to aid the use of insulin already produced by their body. The three main regimens are once-daily basal insulin, twice-daily mix-insulin and basal-bolus therapy.
Basal insulin regimens
When insulin therapy is initiated, it is often because oral treatment cannot maintain blood glucose levels to an acceptable standard. However, as there is no validated or recommended way to determine how much insulin production is present, insulin is often initiated more slowly with only one or two injections per day. NICE recommend initiation of one injection of neutral protamine hagedorn (NPH) insulin a day, which can be titrated to two injections a day if necessary[2]
. The idea of this regimen is to ‘top-up’ endogenous levels, while allowing the pancreas to produce the insulin necessary for mealtime glucose. Often this is achieved by continuing some oral therapies, although with a dose reduction to prevent hypoglycaemia[2]
. This can minimise the dose of insulin needed and reduce weight gain, with a lower risk of hypoglycaemia compared with using insulin alone. This is often done in a specialist setting.
NPH insulin does have a slight peak, unlike analogue insulin, which has a much flatter profile. This can be beneficial, if matched to mealtime glucose peaks (e.g. at breakfast and dinner time), however it also increases the risk of hypoglycaemia if there is no glucose to balance the insulin action (e.g. overnight if given at bedtime).
NICE recommends the use of analogue insulin in the following instances:
- The person needs assistance from a carer or healthcare professional to inject insulin, and use of a long-acting insulin analogue would reduce the frequency of injections from twice to once daily;
- The person’s lifestyle is restricted by recurrent symptomatic hypoglycaemic episodes;
- The person would otherwise need twice daily NPH insulin injections in combination with oral glucose-lowering drugs;
- The person cannot use the device to inject NPH insulin[2]
.
One aspect of these recommendations is that patients must have experienced hypoglycaemia before a switch is considered. This is not acceptable to most practitioners and patients should be assessed for their risk of hypoglycaemia when insulin is initiated. A literature review of hypoglycaemia in type 2 diabetes found a prevalence of 30–56% of mild hypoglycaemia and 0–15% of severe hypoglycaemia (requiring assistance) in patients treated with insulin[3]
. On account of the older age of the population, the duration of the disease, its recognition and the impaired response of patients to hypoglycaemia, these epidemiological studies can be inaccurate. However, it highlights that this is a significant consideration.
These factors significantly increase the risk of hypoglycaemia and poor response to hypoglycaemia[4]
:
- Older age (>65 years)
- Long duration of diabetes (>10 years)
- Multiple comorbidities (e.g. cardiovascular disease, nephropathy, neuropathy)
- Poor diet
- Alcohol consumption
As a result of improved recognition of hypoglycaemia in type 2 diabetes there has been an increase in initiating basal analogue insulin in patients with type 2 diabetes. As shown in ‘Insulin preparations available in the UK’, this choice comes at a significant cost. There is some evidence that analogue insulin decreases the occurrence of hypoglycaemia in type 2 diabetes[5],
[6],
[7]
; however, this is often related to nocturnal hypoglycaemia and using these insulins preferentially has not been shown to be cost-effective or to reduce the overall rates of occurrence[2]
. If risk factors are present then an analogue basal insulin could be considered, but otherwise a patient should be initiated on an NPH insulin as recommended. NICE also recommends the use of an analogue if a patient requires assistance with the administration of insulin, for example from a carer or district nurse[2]
. This is because of their long duration profile and the fact that they do not have to be accurately timed with meals.
It should be noted that Levemir can last up to 20 hours, although it does appear to have a shorter duration than glargine (Lantus, Toujeo, Abasaglar).
Biphasic or mixed insulin regimens
Basal insulin may not be able to maintain good glycaemic control in patients with little endogenous insulin production. This can often be identified by high post-prandial blood glucose levels, which indicate that not enough insulin is available to deal with the glucose load. There has been an increased focus on controlling high post-prandial glucose levels since these have been associated with an increased risk of cardiovascular disease[8],
[9]
.
Mixed insulins are a combination of short-acting and long-acting insulin and allow a more physiological match to glucose intake, without increasing the number of injections required. These are given twice daily and are usually matched to the most significant meals of the day; often breakfast and dinner time (see ‘Insulin profiles for common insulin regimens’). These are available in different proportions of insulin, which allow more specific tailoring to the patient’s requirements. Both human and analogue mixes are available. Human insulin mixes are preferred in type 2 patients because of cost and lack of evidence showing inferiority. However, analogue mixes benefit from faster onset and shorter duration of the rapid-acting portion, which may benefit some patients.
Mixed insulin regimens only suit patients with fairly stable routines. Those that eat a varied diet or miss meals would be at increased risk of hypoglycaemia because of the proportion of rapid and intermediate insulin — once it is given there is nothing that can be done to negate its effects and a lack of mealtime glucose would increase the risk of hypoglycaemia. The duration of action can also not be altered, therefore if a meal is missed or taken early or late, it is likely to impact blood glucose levels significantly. Blood glucose testing is important, since the inclusion of short-acting insulin increases the chance of hypoglycaemia. Patients should not alter doses according to meals because this also alters the amount of long-acting basal insulin, which may result in hyperglycaemia or hypoglycaemia, especially at night.
Basal-bolus regimen
When considering this regimen, the same principles apply in type 2 diabetes as in type 1 diabetes. This regimen carries the highest risk of hypoglycaemia and so patients must commit to regular blood glucose testing. Although this regimen can be used with carb-counting to improve glycaemic control and increase flexibility, it is also used in a fixed-dose regimen for patients who do not have this ability when short-acting insulin is matched to post-prandial glucose excursions. This requires the patient to eat regular, similar meals to allow the healthcare professional to calculate the appropriate doses for patients (see ‘Insulin profiles for common insulin regimens’ for a profile of the basal-bolus [intensive insulin] regimen).
None of the insulin regimens discussed have been demonstrated to be superior. A trial carried out by Holman et al demonstrated that although biphasic and prandial insulin regimes achieved a slightly lower HbA1c, this was accompanied by more cases of hypoglycaemia and increased weight gain[10]
. As a result, the regimen must be chosen on an individual basis, taking into account patient lifestyle, safety and health.
Patient factors
Although the information above aids understanding of the different regimens and reasons for choosing them, the fact remains that insulin therapy is difficult and time-consuming for both patients and healthcare professionals. Patients are often fearful of starting injections and, once initiated, may have difficulty in undertaking the monitoring and maintenance involved. Fear of hypoglycaemia can lead to poor practice (e.g. keeping glucose levels higher than necessary and some patients may start to eat more to match the insulin given), leading to further weight gain and exacerbation of blood glucose control.
An individual’s basal insulin requirement can vary because of physical stress, hormonal changes, physical activity and overall health. Effective insulin regimens are individualised to reflect the patient’s health, goals, lifestyle and ability for self-management. When designing an insulin regimen these must be considered carefully and shared decision making should be the backbone of the consultation process. The following factors should be considered because each one will affect the type of insulin and regimen chosen:
- Patient engagement: patients who are unlikely to be compliant with insulin injections, blood glucose monitoring or who require assistance with administration should be initiated on as few injections as possible;
- Hypoglycaemia risk: patients at high risk of hypoglycaemia should be initiated on a regimen with as low a risk as possible (e.g. once daily basal analogue);
- Fear of injections/needles: this needs to be discussed because there is currently no alternative way of delivering insulin. Injection numbers can be minimised, but patients will need support to deliver insulin.
- Lifestyle: patients with a varied lifestyle may need the flexibility conferred by a basal-bolus regimen, while those who have a fairly fixed lifestyle will be unlikely to need this level of input;
- Diet and alcohol: patients must commit to eating regularly and keeping to recommended alcohol limits for good blood glucose control. Missing meals, having a poor diet or consuming large volumes of alcohol increases the risk of hypoglycaemia;
- Exercise: exercise can mean that insulin regimens need to be flexible or doses need to be tailored, or a patient may need to eat more carbohydrates.
The crucial aspect to avoiding hypoglycaemia is relaxing glycaemic targets – insulin can lead to hypoglycaemia if the fasting blood glucose levels or HbA1c targets are set too low. The risk of hypoglycaemia should be balanced against the risk of complications. The target HbA1c (or blood glucose levels) should be agreed with the individual, taking into account age, risk of hypoglycaemia, comorbidities and life expectancy. It may be appropriate to aim for control of hyperglycaemic symptoms only (i.e. avoiding hypoglycaemia and symptomatic hyperglycaemia).
Initial insulin dosing
Insulin requirements vary greatly and can range from five units to several hundred units a day. Therefore, there is no fixed way to calculate or initiate a specific dose and it is usually done following the classic mantra ‘start low and go slow’. Although long-term glycaemic control is desired, this does not have to be achieved in a matter of days or even weeks. Patient factors, such as body weight, degree of insulin resistance and treatment response, can affect what is needed and must be considered. It is often recommended that patients are initiated on 10 units, or 0.2 units/kg, per day. On average, the eventual daily insulin requirement is 0.5–1.0 unit/kg per day[11]
. Patients who are obese are often more insulin resistant and may need higher doses than 1.0 unit/kg per day. Patients who are also maintained on oral therapies are likely to need lower doses. Adjustment usually occurs in increments of 10% of the original dose, which may be as little as one or two units of insulin[12]
. Insulin requirements in type 2 diabetes change and predominantly increase over time. This is a result of many factors, including weight gain, although it is predominantly believed that residual pancreatic function to produce insulin diminishes with time. As a result, doses must be reviewed and titrated carefully to maximise effectiveness, and use of more complex regimens, such as pre-mixed or basal bolus, may be necessary[13]
.
Newer insulin therapies
Over the past year, a number of newer insulin therapies have been approved, with more anticipated in the coming months. Each has brought with it new difficulties, and consensus groups are considering where and how these insulins can be best used. Tresiba is an ultra-long-acting analogue that offers the opportunity to reduce the occurrence of nocturnal hypoglycaemia. However, it is available at a greatly increased cost (see ‘Insulin preparations available in the UK’), which has resulted in most NHS clinical commissioning groups restricting it for use in patients with type 1 diabetes. Toujeo is a concentrated insulin (300 units/mL rather than the standard 100 units/mL) that also offers a long duration of action and lower hypoglycaemia rates but comes with an increased governance risk if insulin is withdrawn from the prefilled pen. Abasaglar is the first biosimilar insulin to market and offers a cheaper basal analogue insulin. This may prove beneficial in type 2 diabetes where the costs are increasing rapidly but the market is still unsure of the efficacy and risks of biosimilars. New biphasic or mixed insulins are being launched, the latest being Ryzodeg — a combination of Tresiba and Novorapid — which offers a longer basal component than currently available in mixed preparations. It remains to be seen how these insulins will affect the market and the uptake in the type 1 and type 2 populations.
Victoria Ruszala MRPharmS is diabetes and endocrinology group co-chair of the United Kingdom Clinical Pharmacy Association and specialist pharmacist, diabetes and endocrinology, at North Bristol NHS Trust.
Financial and conflicts of interest disclosure: the author has received fees from Sanofi and Janssen for advisory board participation. The author has also received fees for health professional teaching sessions from Astellas, Boehringer/Lilly Alliance, AstraZeneca, the Centre for Pharmacy Postgraduate Education and the UK Clinical Pharmacy Association. No writing assistance was utilised in the production of this manuscript.
- This article was amended on 18 January 2016 to correct spelling mistakes in figure 1. Glycogen was mispelled as glucogen in two instances, and glucagon was mispelled as glucogen in one instance.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] National Institute for Health and Care Excellence. Type 1 diabetes in adults: diagnosis and management. NG17. 2015. Available at: http://www.nice.org.uk/guidance/ng17/resources/type-1-diabetes-in-adults-diagnosis-and-management-1837276469701 (accessed December 2015).
[2] National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NG28. 2015. Available at: http://www.nice.org.uk/guidance/ng28 (accessed January 2016).
[3] Zammit NN & Frier BH. Hypoglycaemia in type 2 diabetes. Diabetes Care 2005;28(12):2948–2961. Available at: http://care.diabetesjournals.org/content/28/12/2948.full.pdf (accessed December 2015).
[4] Akram K, Pedersen-Bjergaard U, Borch-Johnsen K et al. Frequency and risk factors of severe hypoglycemia in insulin-treated type 2 diabetes: a literature survey. J Diabetes Complications 2006;20(6):402–408. doi: 10.1016/j.jdiacomp.2005.08.005
[5] Rosenstock J, Schwartz SL, Clark CM et al. Basal insulin therapy in type 2 diabetes. Diabetes Care 2001;24:631–636. doi: 10.2337/diacare.24.4.631
[6] Hermansen K, Derezinski T, Kim H et al. Treatment with insulin detemir in combination with oral agents is associated with less risk of hypoglycaemia and less weight gain than NPH insulin at comparable levels of glycaemic improvement in people with type 2 diabetes (Abstract). Diabetologia 2004;47(1):A273.
[7] Rosenstock J; The HOE 901/204 Study Investigators Group. Safety and efficacy of insulin glargine (HOE 901) versus NPH insulin in combination with oral treatment in type 2 diabetic patients. Diabet Med 2003;20(7):545–551. doi: 10.1046/j.1464-5491.2003.00999.x
[8] Cavalot F, Petrelli A, Traversa M et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006;91:813–819. doi: 10.1210/jc.2005-1005
[9] Raz I, Wilson PWF, Strojek K et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009;32:381–386. doi: 10.2337/dc08-1671
[10] Holman RR, Thorne KI, Farmer AJ et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357(17):1716–1730. doi: 10.1056/nejmoa075392
[11] Nathan DM, Buse JB, Davidson MB et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32(1):193–203. doi: 10.2337/dc08-9025
[12] Royal College of Nursing. Starting injectable treatment in adults with type 2 diabetes: RCN guidance for nurses (2012). Available at: www.rcn.org.uk (accessed December 2015).
[13] Fonseca VA. Defining and characterizing the progession of type 2 diabetes. Diab Care 2009;32(2):S151–S156. doi: 10.2337/dc09-s301