Nephron / Wikimedia Commons
In this article you will learn:
- How liver disease is classified
- Common signs and symptoms of liver impairment
- How to ensure medicines are used safely in patients with liver impairment
The Global Burden of Disease (GBD) project estimated that there were just over one million deaths globally (around 2% of all deaths) caused by liver cirrhosis in 2010. A further one million deaths were attributable to acute hepatitis and liver cancer[1]
.
Liver disease is the third most common cause of premature death in the UK, with alcohol the most common cause of liver impairment. The prevalence of liver disease in the UK is increasing at a substantially higher rate than other countries in Western Europe (an increase of 62% since 2002), with a four-fold increase in mortality rates since 1970, and an almost five-fold increase in mortality in patients younger than 65 years; by comparison, mortality rates in Western European countries have fallen, with the UK having overtaken France, Spain and Italy in terms of mortality related to liver disease[2]
.
Liver disease can manifest in a broad spectrum of conditions. These can range from mild and self-limiting to severe with high mortality (see ‘Common causes of liver disease’). Patients with liver disease do not necessarily have liver dysfunction, many patients are asymptomatic until they develop complications and there can often be a long time interval between disease occurrence and detection.
Common causes of liver disease
- Alcohol;
- Biliary tract disorders;
- Drugs and toxins (drug-induced liver disease);
- Cancer (hepatocellular carcinoma);
- Leptospirosis or Weil’s disease;
- Immune diseases (e.g. autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis);
- Inherited and metabolic disorders (e.g. Wilson disease, haemochromatosis, glycogen storage disease);
- Non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease (NAFLD);
- Vascular abnormalities (e.g. Budd-Chiari syndrome, veno-occlusive disease (VOD));
- Viral hepatitis — hepatitis A, B, C, D and E.
Classification
Liver disease is classified according to both the pattern of damage seen and time over which the damage occurs. The main patterns of damage are classified as cholestatic or hepatocellular. However, these are not distinct entities and overlap occurs. Acute liver disease is when the history of onset of symptoms does not exceed six months; patients with symptoms of liver disease that last for more than six months have chronic liver disease.
Cholestasis occurs when there is disruption of bile flow between the bile duct and duodenum. Intrahepatic cholestasis affects biliary ductules and is seen in people with primary biliary cirrhosis, in drug-induced liver disease and inflammation. Extrahepatic cholestasis occurs when there is a mechanical obstruction (e.g. inflammation of bile duct, strictures, gallstones). Cholestasis leads to impaired biliary excretion and reduced absorption of fatty substances. Accumulation of bile salts can lead to damage of hepatocytes.
Hepatocellular disease occurs when there is direct injury to hepatocytes (e.g. due to toxins or viruses). Accumulation of fat within hepatocytes is known as steatosis, which can further be categorised as either microvascular or macrovascular. Most people who regularly drink heavily will get a build up of fat in their liver cells. In most cases, this is usually asymptomatic and reversible with abstinence. Hepatitis is inflammation of hepatocytes, which can be acute or chronic and affect only a small area or be widespread.
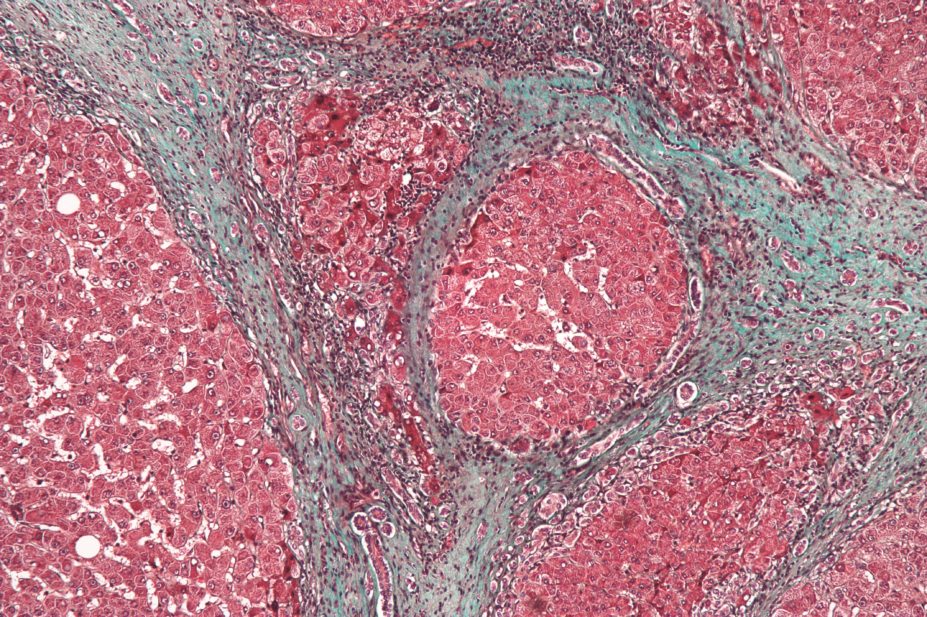
Both cholestasis and hepatocellular disease can lead to fibrosis and cirrhosis. Fibrosis occurs when there is persistent, extensive hepatocyte damage, leading to active deposition of collagen and formation of scar tissue. Cirrhosis is a progressive disease defined as fibrosis and nodular regeneration, resulting in disruption of the normal architecture of the liver. Cirrhosis is also associated with a reduction in hepatic cell mass, and a corresponding decrease in the functional capacity of the liver. It can be classified as compensated cirrhosis (the liver is able to carry out most of its functions), or decompensated (the liver is extensively scarred and unable to carry out required functions).
Signs and symptoms of liver disease include:
• Jaundice
• Ascites and spontaneous bacterial peritonitis
• Bruising and bleeding
• Portal hypertension and varices
• Encephalopathy
• Pale stools and dark urine
• Gynaecomastia
• Fatty stools
• Spider naevi
• Finger clubbing
• Pruritus.
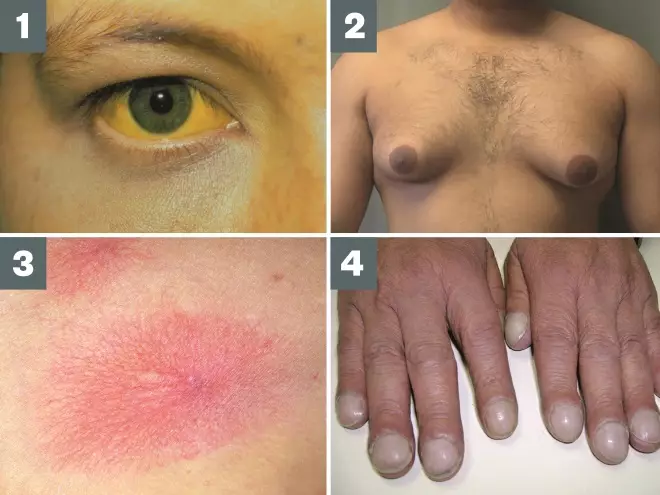
Source: Science Photo Libray / Alamy / Wikimedia Commons
1. Jaundice is the yellowing of the skin and whites of the eyes, caused by the build-up of bilirubin
2. Gynaecomastia is the enlargement of male breast tissue; it has a wide range of causes and occurs in at least one third of all men during their lifetime
3. Spider naevi are common and present as a red spot with radiating extensions
4. Finger clubbing is a sign often associated with conditions affecting the lungs, heart or liver
Assessment
Several factors are taken into account when assessing a patient’s liver function, including cause of impairment, classification of liver disease (e.g. cirrhotic compared with non-cirrhotic), signs and symptoms and liver functions tests (LFTs).
LFTs are assessed via a blood sample (see ‘Common liver function tests’). It is important to look at results not just in isolation but at the trend over time; generally, a test is considered ‘abnormal’ if the result is two-times the upper limit of the normal range[3]
. Abnormal LFTs are not necessarily because of liver dysfunction; none of the liver function tests are specific for the liver and therefore derangement is not necessarily related to liver function. For example, an elevated bilirubin could be caused by haemolytic anaemia.
Usually, patients with liver dysfunction will have at least two deranged LFTs, although it is possible for patients with dysfunction, including those with cirrhosis, to have normal LFTs. Other investigations that may be carried out to assess liver function include ultrasounds, computed tomography (CT) scans, fibroscans and liver biopsies.
Common liver function tests
| Liver function test | Usual range | Information |
|---|---|---|
Albumin | 34–45g/l |
|
Alkaline phosphatase (ALP) | 30–300 international units/l |
|
Bilirubin | 5–21μmol/l |
|
Gamma-glutamyltransferase (GGT) | 0–50 international units/l |
|
International normalised ratio (INR) or prothrombin time (PT) | INR < 1.2 PT < 16 secs |
|
Transaminases: aspartate aminotransferase (AST) alanine aminotransferase (ALT) | 0–40 international units/l
|
|
Knowing the signs and symptoms of liver disease that the patient is exhibiting is also important when assessing the patient’s overall liver function.
There is little information available to help guide drug dosing in liver disease as there is no standard measure that can be used. Overall, the following patient points should be considered:
- How severe is the patient’s liver disease (e.g. cirrhotic compared or non-cirrhotic, compensated or decompensated)? A patient with decompensated cirrhosis is likely to have altered drug handling and therefore more caution is required when it comes to both drug selection and dose used.
- What is their underlying liver diagnosis?
- What is the trend in the patient’s LFTs? What is their synthetic function like (albumin, bilirubin, international normalised ratio (INR))?
- What signs and symptoms of liver disease is the patient exhibiting?
The Child-Pugh scoring system (see ‘Child-Pugh scoring system’) is often used to make recommendations in the summary of product characteristics for individual drugs; however, this system was developed to predict disease severity and patient outcome, not drug clearance[3]
,
[4]
.
Child-Pugh scoring system
| Parameter/Score | 1 | 2 | 3 |
|---|---|---|---|
The Child-Pugh scoring system assesses presence and degree of ascites and encephalopathy, serum albumin and bilirubin. Each parameter is given a score of 1–3 and the overall score gives the patient a classification of A, B or C. Child-Pugh A (well compensated liver) = 5–6 points Child-Pugh B (significant dysfunction) = 7–9 points Child-Pugh C (decompensated) = 10–15 points | |||
Ascites | None | Mild–moderate | Severe or intractable |
Encephalopathy | None | Grade 1–2 | Grade 3–4 |
Bilirubin (μmol/l) | < 35 | 35–50 | > 50 |
Albumin (g/l) | > 35 | 28–35 | < 28 |
INR | < 1.7 | 1.8–2.3 | > 2.3 |
Albumin, INR, prothrombin time (PT) and bilirubin are markers of the synthetic and excretory function of the liver. Unfortunately, there are limited studies in correlating these parameters with drug dosing.
Cholestasis can result in drugs that are highly lipophilic (e.g. nifedipine, amitriptyline) being less well absorbed as they are dependent on the excretion of bile salts. This can lead to reduced plasma concentrations and clinical efficacy[3]
. However, the clinical significance of this is likely to be minimal for most patients.
Medicines that undergo enterohepatic recirculation (e.g. oestrogens, methodone) are also likely to be less effective in patients with cholestasis, as there is likely to be less recirculation and potentially reduced exposure[3]
. The clinical significance of this is unclear.
Conversely, medicines that undergo extensive biliary clearance (e.g. vinca alkaloids, doxorubicin) are likely to remain in the body longer, resulting in increased exposure and clinical effect. This can be clinically significant and therefore dose reduction recommendations can be made on the basis of bilirubin levels[5]
.
Bilirubin can also compete with medicines for protein binding sites, displacing them from their binding sites and therefore further increasing unbound drug levels and exposure.
Decreased albumin levels (hypoalbuminaemia) can increase the clinical effect of drugs. As many medicines and molecules bind to albumin, this results in increased levels of free medicines available to act, and therefore an increased clinical effect.
This is not clinically significant for most medicines, although it can increase the clinical effect of highly protein-bound medicines, such as phenytoin. This is important in therapeutic drug monitoring because usually total phenytoin levels are reported (i.e. both bound and unbound fractions), and this must be corrected for patients with a low albumin level as they will have a higher concentration of unbound drug.
A calculation can be performed to correct this as follows[6]
:
Corrected concentration = Measured concentration μmol/l / (0.9 x albumin g/l / 44) + 0.1
Other markers, including INR and PT, aspartate aminotransferase (AST) and a
lanine aminotransferase (ALT)
, Alkaline phosphatase (ALP) and gamma-glutamyltransferase (GGT), provide a general marker of liver function but are not generally used to guide dosing in liver disease.
Medicines that can be affected by liver disease
| Types of medicines | Examples | Impact in liver disease |
Constipating medicines
| Opioid analgesics Sedating antihistamines Antimuscarinic drugs | Precipitate or worsen encephalopathy |
Medicines that cause gastrointestinal ulceration | NSAIDs Aspirin Bisphosphonates | Increased risk of gastrointestinal mucosal damage and bleeding |
Sedating medicines | Opioid analgesics Benzodiazepines Hypnotics | Precipitate or worsen encephalopathy |
Anticoagulants, antiplatelets and other medicines that can cause bleeding | Warfarin Aspirin Clopidogrel NSAIDS | Increased risk of bleeding on account of underlying coagulopathy, low platelets |
Medicines that can affect fluid-electrolyte balance | Diuretics | Precipitate or worsen encephalopathy |
Medicines with a high sodium content | Antacids Soluble tablets (e.g. soluble paracetmol) Some intravenous preparations | Precipitate or worsen ascites |
Medicines that are nephrotoxic | NSAIDS Aminoglycosides | Precipitate or worsen heptorenal syndrome |
Administration
The oral route is generally preferred in patients with liver disease, although modified-release or long-acting preparations should be avoided as they can accumulate. Intramuscular injections should be avoided in patients with liver disease and coagulopathy as they can lead to the development of a haematoma at the injection site.
Topical preparations and patches may cause increased irritation to the skin in patients with liver disease.
If rectal preparations are being used, the patient should be assessed for the potential presence of rectal varices and the increased risk of bleeding.
Pharmacokinetics
Liver disease will influence pharmacokinetic paraÂmeters such as absorption, distribution, metabolism and excretion of a medicine. However, the capacity of the liver is so great that liver disease must usually be extensive before effects on drug handling become clinically significant. The extent of these changes is frequently complex, unpredictable and drug specific; as a result, estimates of dose reductions are difficult[3],[7]
.
Absorption may be reduced in the presence of ascites, although this can be difficult to predict from pharmacokinetic data; this occurs with furosemide[3],[5]
. Lipid-soluble medicines may also be less well absorbed.
Distribution is important, because if a drug is water soluble then it may distribute into ascites, which can mean that a lower concentration is achieved in other areas of the body and a greater initial dose may be required[3],[5]
.
Metabolism in the liver can be affected by liver blood flow and functioning cell mass, which are difficult to assess in practice. Medicines that undergo hepatic metabolism then a reduced hepatic cell mass caused by cirrhosis may lead to a subsequent reduction in drug-metabolising enzymes, the accumulation of active drug, and the potential for an enhanced response and increased adverse effects[3]
.
Cirrhosis can cause a reduction in intrahepatic blood flow and the development of portosystemic shunts, which divert blood from the liver to the systemic circulation. This can result in the increased bioavailability of medicines that are highly extracted on the first pass through the liver, which can cause an increase in a medicine’s peak plasma concentration and a prolonged half-life. This means patients may require a reduced dose or an increase in time between doses. For example, propranolol is commonly used in patients with liver disease to treat portal hypertension, but at doses much lower (commonly 20–40mg orally once or twice a day) than to treat cardiovascular disease in patients with normal liver function. Other high extraction medicines include morphine, sertraline and sumatriptan.
Reduced functional Âcapacity and hence delayed elimination from the systemic circulation will also prolong the half-life of medicines with low hepatic extraction, which are dependent on the functional capacity of the liver for their clearance. In such cases, adjustment of the dosing interval will be necessary to avoid toxicity on repeated dosing. There is no set increase in dose interval and a decision will have to be made on an individual basis. Examples of low extraction ratio medicines include citalopram and theophylline.
Excretion can be impaired in liver disease. It is also important to note that renal excretion can be affected in patients with liver disease, and creatinine clearance may overestimate the glomerular filtration rate in patients with cirrhosis[3],[5],[7]
. Patients may also have hepatorenal syndrome.
Pharmacodynamics
Pharmacodynamic variations associated with liver disease can affect a patient’s response to therapy. Patients may be at risk of increased toxicity and side effects, exaggerated response or reduced response.
Cerebral sensitivity and tissue responsiveness to medicines with sedative and hypnotic effects is increased in patients with liver disease[8]
. Medicines with cerebral depressant activity (e.g. opioid analgesics, benzodiazepines) should be carefully monitored if used in patients with severe liver disease, and possibly prescribed at a reduced dose, as there is a risk of precipitating or worsening hepatic encephalopathy. In encephalopathy, the brain becomes more sensitive to the sedating effects of a medicine on account of altered blood-brain permeability, cerebral blood flow and receptor sensitivity[3]
.
Side effects
Medicines with side effects that could potentially affect hepatic function should be avoided in patients with liver impairment. This includes medicines that can affect clotting (due to the risk of coagulopathy in liver disease), and those that can cause fluid retention and electrolyte imbalances, which can lead to increased ascites and encephalopathy.
Medicines that can cause constipation can also precipitate or worsen encephalopathy in patients with liver disease on account of the accumulation of toxic waste products that can cross the blood-brain barrier. In these cases, an alternative medicine that causes less constipation should be used, or a laxative such as lactulose (which is also used to prevent and treat encephalopathy) should be co-prescribed.
Non-steroidal anti-inflammatory drugs (NSAIDs) are contraindicated in patients with any degree of liver cirrhosis due to their potential side effects (e.g. fluid retention and electroÂlyte abnormalities; gastrointestinal ulceration and bleeding; inhibition of platelet aggregation, and a reduction in glomerular filtration rate). NSAIDs have also been rarely associated with hepatoÂtoxicity[3]
.
It is also important to take into account the sodium content of drug formulations; for patients with ascites, sodium intake should ideally be restricted to approximately 2g/day (90 mmol) as a higher intake than this can exacerbate problems relating to fluid retention. For example, antacids are frequently prescribed for patients with liver disease and can often contain high amounts of sodium; in these patients, a low sodium antacid (e.g. Maalox, which contains <1mmol sodium per 10ml dose) should be prescribed.
Drug-induced liver disease
More than 900 drugs, toxins, and herbs have been reported to cause liver injury, and this is the most common reason cited for withdrawal of an approved drug (e.g. troglitazone)[9]
.
Risk factors that may predispose patients to drug-induced liver disease include sex (it tends to be more common in females), age, genetics, concurrent diseases (e.g. obesity, diabetes, co-infection with HIV) and polypharmacy[3]
. Withdrawal of the drug generally results in resolution of the liver disease, although this is not always the case.
Drug-induced liver disease can be broadly categorised into intrinsic reactions and idiosyncratic reactions. Intrinsic reactions (such as paracetamol overdose) are predictable, reproducible, dose dependent and tend to occur rapidly (e.g. within hours). They tend to cause necrosis and acute liver failure.
Idiosyncratic reactions are not predictable, not reproducible, do not depend on dose and tend to take longer to occur (e.g. weeks to months). They can result from metabolic idiosyncrasy or hypersensitivity reaction, and can cause any type of liver injury, resulting in signs and symptoms such as increased liver function tests, jaundice, fever, rash and eosinophilia.
Potential idiosyncratic reactions, and their causative medicines, include:
- Acute liver failure — allopurinol, aspirin, cyclophosphamide, NSAIDs;
- Cholestasis — warfarin, azathioprine, carbimazole, oral contraceptive pill, flucloxacillin;
- Fibrosis and cirrhosis — methotrexate;
- Acute hepatitis — phenytoin, isoniazid;
- Chronic hepatitis — isoniazid;
- Steatosis — amiodarone, corticosteroids, total parenteral nutrition;
- Vascular disorders — oral contraceptive pill, azathioprine.
Sarah Knighton is the pharmacy team leader for liver and private patient services at King’s College Hospital, London.
References
[1] Mokdad AA, Lopez AD, Shahraz S et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 2014;12:145.
[2] Williams R, Aspinall R, Bellis M et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. The Lancet 2014;9958:1953–1957.
[3] North-Lewis P (ed). Drugs and the Liver — A Guide to Drug Handling in Liver Dysfunction. London: Pharmaceutical Press 2008.
[4] Johnson PJ & MacFarlane IG. The Laboratory Investigation of Liver Disease. London: Bailliere Tindall 1989.
[5] Delco C, Tchambaz L, Schlienger R et al. Dose adjustment in patients with liver disease. Drug Safety 2005;28:529–545.
[6] Winter ME. Basic Clinical Pharmacokinetics 4th Ed. Baltimore: Lippincott Williams & Wilkins 2004.
[7] Verbeeck RK. Pharmacokinetics and dosage adjustments in patients with hepatic dysfunction. Eur J Clin Pharmacol 2008; 64:1147–1161.
[8] Westphal JF & Brogard JM. Drug administration in chronic liver disease. Drug Safety 1997;17:47–73.
[9] Mehta N, Ozick LA, Gbadehan E et al. Drug-induced Hepatotoxicity. Medscape Drugs and Diseases.