Shutterstock.com
Acute upper gastrointestinal bleeding (AUGIB) is defined as bleeding from the upper gastrointestinal tract, normally from the oesophagus, stomach or duodenum[1]
. Its underlying causes most commonly include peptic ulcer disease and oesophageal or gastric varices. It is a common medical emergency with an estimated UK incidence of 134 cases per 100,000 people[1]
and has a mortality rate of 10%[2]
. In the UK, it is estimated to cause 9,000 deaths annually[3]
, although death in the absence of comorbidities and old age is unusual. The majority of patients with AUGIB will present to hospital; therefore, this article focuses on hospital assessment and provides an overview of the available treatments.
Risk factors
Taking certain medicines can be contributory factors for AUGIB. Non-steroidal anti-inflammatory drugs (NSAIDs) and aspirin (a salicylate) can cause peptic ulcer disease, with patients who are taking NSAIDs having a four-fold increase in the risk of ulcer complications compared with those who do not take NSAIDs[4]
.
The risk of ulcer complications (see Box) is progressive depending on the number of risk factors present. The most important risk factors are a history of ulcer complications and advancing age, particularly being aged over 75 years. Corticosteroids alone are an insignificant ulcer risk, but potentiate ulcer risk when added to NSAIDs, particularly in daily doses of at least 10mg prednisolone[5]
.
NSAIDs differ in their propensity to cause ulceration, with ibuprofen and diclofenac considered to have the lowest risk, and ketoprofen and azapropazone the highest risk. The risk also increases with higher doses of NSAIDs[6]
.
Low-dose aspirin (75mg/day) alone increases the risk of ulcer bleeding by around two fold and dual antiplatelet use increases this risk to four fold. Other antiplatelets and anticoagulants also increase the risk of bleeding[7]
, as well as the use of selective serotonin re-uptake inhibitors (SSRIs), owing to the antiplatelet effect of serotonin[8]
.
Box: Risk factors for non-steroidal anti-inflammatory drug ulcers
- Age greater than 65 years;
- Previous peptic ulceration/bleeding;
- High dose of non-steroidal anti-inflammatory drug (NSAID) or more than one NSAID (including aspirin);
- Short-term history of NSAID use (<1 month);
- Concomitant corticosteroid or anticoagulant use;
- Cardiovascular disease.
Presentation
AUGIB should be suspected in patients that present with any of the following symptoms: the production of dark sticky faeces (melaena); vomiting blood (haematemesis); coffee ground vomiting, or an unexplained drop in haemoglobin. Patients may present with hypovolaemia (a state of decreased blood volume; more specifically, decrease in volume of blood plasma) with low blood pressure and tachycardia.
Diagnosis
A diagnosis of AUGIB is confirmed by endoscopy. The National Institute for Health and Care Excellence (NICE) recommends that all clinically unstable patients with severe AUGIB should receive endoscopy immediately after appropriate resuscitation, while all other patients should receive endoscopy within 24 hours[9]
,[10]
. Endoscopy at an early stage identifies the cause of bleeding in most cases and allows therapeutic interventions to be undertaken to stop the bleeding. A 2015 report from the National Confidential Enquiry into Patient Outcome and Death (NCEPOD) stated that 32% of hospitals admitting gastrointestinal (GI) bleed patients did not have a 24-hour endoscopy service[9]
. Their recommendations were that hospitals should either provide this service on site, or set up formal networks to ensure access for patients[9]
. A cost-effectiveness analysis carried out by NICE suggested that providing daily endoscopy lists on site would be cost effective for units seeing more than 330 patient cases each year[10]
.
A number of risk scoring systems are available for assessing the severity of AUGIB (see Table 1 and Table 2). The Blatchford score predicts the vneed for endoscopic intervention[11]
; patients with a score of 0 can be considered for early discharge without an acute endoscopy (Table 1), while the Rockall score predicts mortality (Table 2)[12]
. Both scoring systems are recommended for use by NICE[10]
.
| Table 1: Blatchford score for predicting need for endoscopic intervention | ||
| Risk factor | Score* | |
| Urea (mmol/L) | 6.5 – 8.0 | 2 |
| 8.0 – 10.0 | 3 | |
| 10.0 – 25 | 4 | |
| >25 | 6 | |
| Haemoglobin (g/L) — Men | 12.0 – 12.9 | 1 |
| 10.0 – 11.9 | 3 | |
| <10.0 | 6 | |
| Haemoglobin (g/L) — Women | 10.0 – 11.9 | 1 |
| <10.0 | 6 | |
| Systolic blood pressure (mmHg) | 100 – 109 | 1 |
| 90 – 99 | 2 | |
| <90 | 3 | |
| Other markers | Pulse ≥100 (per minute) | 1 |
| Presentation with melaena | 1 | |
| Presentation with syncope | 2 | |
| Hepatic disease | 2 | |
| Cardiac failure | 2 | |
| *Add scores for each risk factor to give a total score. A score of 0 is associated with a low risk of the need for endoscopic intervention, while scores of 6 or more are associated with a greater than 50% risk of needing an endoscopic intervention. Source: Jiang HY, Chem HZ, Hu XJ et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2015;13:42–50 | ||
| Table 2: Rockall score for risk of rebleeding or death in acute upper gastrointestinal bleeding | ||||
| Variable | Score 0 | Score 1 | Score 2 | Score 3 |
| Age (years) | <60 | 60 – 79 | >80 | |
| Shock | No shock | Pulse >100 beats per minute SBP >100mmHg | SBP <100mmHg | |
| Comorbidity | Nil major | CHF, IHD major morbidity | Kidney failure, liver failure, metastatic cancer | |
| Diagnosis | Mallory-Weiss Tear | GI malignancy | ||
| Evidence of bleeding | None | Blood, adherent clot, spurting vessel | ||
CHF: chronic heart failure; GI: gastrointestinal; IHD: ischaemic heart disease; SBP: systolic blood pressure. Add score for each risk factor to give total score. A score of <3 carries good prognosis but a total score of >8 carries a high risk of mortality. National Confidential Enquiry into Patient Outcome and Death (NCEPOD). | ||||
Pre-endoscopic management
In patients experiencing major bleeding, the immediate priority is to resuscitate with crystalloids and/or blood products in order to stabilise the patient prior to endoscopy. Local protocols for massive haemorrhage should be followed, which may include giving platelets and clotting factor as well as blood transfusions. For less severe GI bleeds, the threshold for transfusion is less clear, with some concerns that a liberal transfusion policy may result in poorer outcomes.
A randomised controlled trial (RCT) by Villaneuva et al. that compared a restrictive red blood cell transfusion strategy (target haemoglobin, 7–9g/dL) with a more liberal transfusion strategy (target haemoglobin, 9–11g/dL) found a significantly improved six-week survival (95% versus 91%; hazard ratio [HR]: 0.55; 95% confidence interval [CI]: 0.33–0.92) and reduced rebleeding (10% vs. 16%; HR: 0.68; 95% CI: 0.47–0.98) with the restrictive strategy[13]
.
A UK pragmatic cluster RCT found no significant difference in clinical outcomes between restrictive (80g/L) and liberal (100g/L) strategies; however, this was a feasibility study and was not adequately powered to detect such outcomes[14]
. The European Society of Gastrointestinal Endoscopy guidelines currently recommend a restrictive strategy that aims for a target haemoglobin of between 7g/dL and 9g/dL[15]
. NICE also recommends that a platelet transfusion is given to patients who are actively bleeding if platelets are less than 50 x 109/L[10]
.
Patients who are taking warfarin and actively bleeding should have their anticoagulation reversed using a combination of prothrombin complex and intravenous (IV) vitamin K 5mg. Vitamin K alone is insufficient for acute bleeding as it can take up to 12 hours for full reversal of anticoagulation. Fresh frozen plasma is inferior to prothrombin complex and should only be used if prothrombin complex is unavailable[16]
.
The short half-life of direct oral anticoagulants (DOACs) means that in the absence of significant kidney impairment the effects should be short-lived; however, they should be withheld prior to endoscopy. If the patient has ingested a DOAC within the past two hours, activated charcoal should be considered.
For dabigatran, the antidote idarucizumab can be given for life-threatening or uncontrolled bleeding — the recommended dose is 5g given intravenously as two 2.5g consecutive infusions over 5–10 minutes each or as a bolus injection. A further 5g dose can be given if there are prolonged clotting times as well as either further clinically relevant bleeding, or if potential rebleeding would be life threatening, or if patients require a second emergency surgery/urgent procedure. Idarucizumab is a humanised monoclonal antibody fragment that binds to dabigatran with very high affinity, approximately 300-fold more potent than the binding affinity of dabigatran for thrombin. Idarucizumab potently and specifically binds to dabigatran and its metabolites and neutralises their anticoagulant effect.
For patients taking anti-Xa inhibitors (apixaban, edoxaban, and rivaroxaban) there are no specific antidotes available. In continuing haemorrhage or major bleeding, prothrombin complex concentrate can be given, although the evidence for this is limited to animal and healthy volunteer studies[17]
.
NSAIDs and antiplatelets are normally withheld pending endoscopy for AUGIB.
Pharmacological agents pre-endoscopy
Non-variceal bleeding
Proton pump inhibitors (PPIs) are often given empirically prior to endoscopy, but their use prior to endoscopic diagnosis has no effect on patient-orientated outcomes of mortality, rebleeding rates, surgery or blood transfusions[18]
and NICE specifically advises against their use. Pre-endoscopic use, however, significantly reduces the requirement for endoscopic therapy (8.6% vs. 11.7%; odds ratio [OR]: 0.68; 95% CI: 0.50–0.93), and European guidelines support pre-endoscopic use on the basis that the reduction in endoscopic treatment and reduction in length of stay is cost effective[15]
.
Presence of blood and clots may impair visualisation of bleeding sources during endoscopy; therefore, there is a potential role for the use of pro-kinetics to improve gastric emptying prior to endoscopy. The agent with the best evidence is erythromycin, for which a meta-analysis of studies showed that infusion prior to endoscopy improves mucosal visualisation (OR: 3.43; 95% CI: 1.81–6.50), decreases the need for a second endoscopy (OR: 0.47; 95% CI: 0.26−0.83), units transfused (−0.41 units; 95% CI: −0.82 to −0.01) and duration of hospital stay (weighted mean difference −1.51 days; 95% CI: −2.45 to −0.56)[19]
.
The regimen most commonly used is 250mg given intravenously 30–120 minutes prior to endoscopy in patients with evidence of active bleeding. However, the impact of single dose macrolide use on Helicobacter pylori resistance is currently unknown.
Routine use of IV tranexamic acid as an anti-fibrinolytic is not currently recommended. A Cochrane review has demonstrated a reduction in mortality; however, there was no effect on rebleeding, blood use or surgery, and the majority of the trials included were carried out prior to the advent of PPIs and endoscopic therapy[20]
. Results from the ongoing Haemorrhage Alleviation With Tranexamic Acid-Intestinal System trial should help inform practice in this area[21]
.
Variceal bleeding
The two classes of drugs studied in controlling acute variceal bleeding are vasopressin and its analogues (including terlipressin), and somatostatin and its analogues (including octreotide). They act as vasoconstrictors, reducing splanchnic blood flow and portal vein flow and pressure.
Patients with suspected variceal bleeding should be started on antibiotics and terlipressin. Bacterial infections are a frequent complication in patients with cirrhosis and AUGIB, and a Cochrane review found antibiotic prophylaxis was associated with improved mortality (relative risk [RR]: 0.79; 95% CI: 0.63–0.98), and a reduction in rebleeding (RR: 0.53; 95% CI: 0.38–0.74) and days of hospitalisation (MD –1.91; 95% CI: –3.80 to –0.02)[22]
. Broad-spectrum antibiotics, such as cephalosporins, are normally used but choice of antibiotics should be dictated by local resistance patterns and availability. If variceal bleeding is confirmed, the most appropriate length of course is unclear, with regimens varying from single dose to ten days, although in the absence of active infection a shorter course of 48–72 hours would seem appropriate to minimise unnecessary antibiotic exposure.
Terlipressin given as 1–2mg every 4–6 hours is the agent of choice as it is the only agent that has been shown in a meta-analysis to reduce mortality (RR: 0.66; 95% CI: 0.49 to 0.88)[23]
. Owing to its vasoconstrictive properties, particular caution is advised when it is used in patients with ischaemic heart disease and vascular disease.
If terlipressin is not tolerated, an unlicensed alternative is available — octreotide can be given as 50µg bolus followed by 25–50µg/hour infusion. A Cochrane review comparing octretide with terlipressin showed no difference in mortality (RR: 1.20; 95% CI: 0.66 to 2.15)[23]
, although octreotide itself has not been demonstrated to reduce mortality in comparison to placebo. NICE advises to stop treatment after definitive haemostasis has been achieved, or after five days, unless there is another ongoing indication for its use (e.g. hepatorenal syndrome)[10]
.
Endoscopic therapy
Non-variceal bleeding
When peptic ulcer disease is discovered at endoscopy, high-risk lesions, such as a visible blood vessel, active bleeding or adherent clot, can be treated endoscopically to achieve haemostasis and such treatment has been shown to reduce mortality, rebleeding risk and surgery. First-line therapies include injection therapy with adrenaline (1 in 10,000 injected into and around the bleeding point), thermal therapy (heater probe or argon plasma coagulation), or mechanical therapy with clips.
NICE guidelines recommend that adrenaline should be used in combination with thermal therapy[10]
, clips or fibrin/thrombin, as dual therapy is superior in reducing rebleeding (10.6% vs. 18.4%; OR: 0.53; 95% CI: 0.40–0.69), emergency surgery (7.6% vs. 11.3%; OR: 0.64; 95% CI: 0.46–0.90), and mortality (2.6% vs. 5.1%; OR: 0.51; 95% CI: 0.31–0.84)[24]
. Alternatively, NICE suggests clips can be used alone[10]
.
Spray therapy with a topical haemostatic agent is emerging as an alternative, particularly where lesions are difficult to access to apply conventional therapy, although RCTs directly comparing them with conventional therapy are required to further clarify their role[15]
.
Variceal bleeding
For bleeding varices, therapeutic options are banding or sclerotherapy. First-line therapy for oesophageal varices is band ligation, where the endoscope is used to suck a loop of varix into a plastic tube. A rubber band is then deployed over the loop leading to thrombosis of the varix. A meta-analysis of seven trials comparing banding to sclerotherapy found it superior in terms of reducing rebleeding (OR: 0.52; 95% CI: 0.37–0.74) and mortality (OR: 0.67; 95% CI: 0.46–0.98)[25]
. For gastric varices there is less evidence to guide choice of therapy; however, NICE currently recommends sclerotherapy with cyanoacrylate[10]
.
Alternative sclerosing agents that have been used include ethanolamine, sodium tetradecyl sulfate and absolute alcohol, and another alternative for gastric varices is an injection of thrombin.
Balloon tamponade using a Sengstaken Blakemore tube (see Figure) is reserved for when haemostasis cannot be achieved. Its use is associated with a high rebleeding rate once the balloon is deflated, so it is mainly used as a bridge until banding or sclerotherapy can be repeated. It is also poorly tolerated, requiring sedation and intubation; therefore, it should be managed in an intensive care setting.
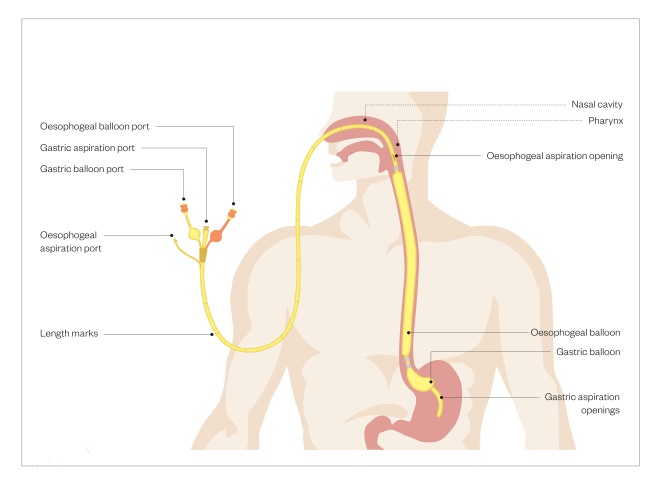
Figure: Sengstaken Blakemore tube
Balloon tamponade can be used when haemostasis cannot be achieved for variceal bleeding. Owing to associated high bleeding rates with its use, it is mainly used as a bridge until banding or sclerotherapy can be repeated
Failure of endoscopic therapy
Non-variceal bleeding
If endoscopic therapy fails to control therapy at initial or repeat endoscopy, options include offering surgery or interventional radiology, where the bleeding source is first identified using computed tomography angiography, and then the relevant artery branch is embolised. Although a prospective audit showed higher mortality after surgery compared with embolisation[26]
, there are no RCTs directly comparing the two and the lack of centres offering a 24-hour interventional radiology service means that the choice of therapy is currently driven by availability. The 2015 NCEPOD report recommended that centres admitting cases of AUGIB should either have on-site access to interventional radiology or be covered by a formal network[9]
.
Variceal bleeding
Should bleeding not be controlled by either banding or sclerotherapy, surgically fitting a transjugular intrahepatic portosystemic shunt is an alternative option, where a stent is placed radiologically between the portal and hepatic veins. This achieves decompression of the portal vein system, but carries a risk of hepatic encephalopathy.
Post-endoscopic management
Non-variceal bleeding
Gastric acid inhibits clot formation; therefore, if intragastric pH is maintained above 6 during the first 3 days after the initial bleed, there is increased opportunity for clot stabilisation and haemostasis. Results from a Cochrane meta-analysis suggest that PPIs significantly reduce rebleeding rates 10.6% vs. 17.3% (OR: 0.49; 95% CI: 0.37–0.65) and surgery 6.1% vs. 9.3% (OR: 0.61; 95% CI: 0.48–0.78)[27]
and are recommended by NICE[10]
. The optimal dose and route of PPI, however, is less clear for this indication. A reduction in mortality has been observed in high-risk patients when continuous IV PPI therapy is given (80mg bolus omeprazole, pantoprazole or esomeprazole followed by 8mg/hour for 72 hours) following endoscopic haemostasis[28]
, but a more recent meta-analysis has suggested that intermittent PPI is non-inferior in terms of rebleeding rates[29]
. European guidelines now suggest intermittent oral or IV twice daily high-dose PPI as an alternative to the continuous intravenous regime[15]
.
As with uncomplicated ulcer disease, detection and eradication of H. pylori is important for reducing recurrent disease. There is a reduced sensitivity of testing for H. pylori in the context of AUGIB, therefore European guidelines recommend retesting for H.pylori if the initial test in the acute setting is negative. Eradication of H. pylori reduces the rate of rebleeding to a greater extent than non-eradication (OR: 0.16; 95% CI: 0.09–0.30; number needed to treat [NNT]: 7) or long-term antisecretory therapy (OR: 0.14; 95% CI: 0.05–0.43; NNT: 20)[30]
. Following successfulH. pylori eradication and healing of the ulcer, there is no need to continue maintenance antisecretory therapy beyond four weeks unless required for prophylaxis of ulcer complications in patients who are continuing to take aspirin or NSAIDs[31]
.
Patients receiving aspirin monotherapy for secondary prevention of vascular events should continue aspirin once bleeding is controlled because, although the risk of rebleeding is increased by restarting aspirin early, as opposed to waiting a month, the overall risk of mortality at 30 days is lower (10.3% vs. 1.3%; P = 0.001)[32]
. In these patients, the continuation of a long-term PPI is of benefit in the prevention of recurrent bleeding[33]
.
If aspirin is being taken for primary prevention, the risk of bleeding may outweigh the benefits and stopping aspirin permanently should be considered. Clopidogrel alone is not a safer alternative than aspirin and a PPI in terms of prevention of recurrent ulcer bleeding. For patients on other antiplatelets or dual antiplatelet therapy (DAPT), the balance of risk and benefit is less clear and is likely to depend on the indication (e.g. acute coronary syndrome or for cardiac stent) and how long ago the procedure or event occurred. Close collaboration between gastroenterology and cardiology teams is recommended. If DAPT is continued, gastroprotection with a PPI is recommended[28]
. The conversion of clopidogrel to its active form is reliant on CYP2C19 and the inhibition of this activity by omeprazole and esomeprazole may be of concern; however, the clinical relevance of this is unclear. Recent guidance from the Medicines and Healthcare Products Regulatory Agency advises the avoidance of concurrent use of esomeprazole and omeprazole with clopidogrel, but not other PPIs, acknowledging the lack of clear evidence to support an interaction and the potential benefits of concurrent use in reducing risk of GI bleeding[34]
.
For patients who were receiving anticoagulation, the ongoing indication for anticoagulation should be reviewed. For patients with atrial fibrillation, the risk of stroke and bleeding can be recalculated using the CHA2 DS2 -VASC and HAS-BLED scores, respectively, and used to facilitate a patient-centred discussion over their preferences for continuing or stopping anticoagulation (see Table 3 and Table 4). For high-risk patients, such as those with metallic heart valves or recent pulmonary embolism/deep vein thrombosis, bridging with low molecular weight heparin or unfractionated heparin can be considered.
| Table 3: Calculating CHA2 DS2 -VASC score for stroke risk | |
| Add scores for each risk factor. Consult www.chadsvasc.org to determine adjusted stroke rate risk. | |
| Risk factors | Score |
| Congestive heart failure | 1 |
| Hypertension | 1 |
| Age ≥75 years | 2 |
| Age 65–74 years | 1 |
| Diabetes mellitus | 1 |
| Stroke, transient ischaemic attack or thromboembolism | 2 |
| Vascular disease | 1 |
| Female | 1 |
| Table 4: Calculating HAS-BLED score for bleeding risk | |
| Source: www.chadsvasc.org | |
| Clinical characteristic | Points awarded |
| Uncontrolled hypertension | 1 |
| Abnormal liver function | 1 |
| Abnormal kidney function | 1 |
| Previous history of stroke | 1 |
| Bleeding history or predisposition | 1 |
| Labile international normalised ratio | 1 |
| Elderly (aged ≥56 years) | 1 |
| Antiplatelets or non-steroidal anti-inflammatory drugs | 1 |
| Alcohol use (over eight units per week) | 1 |
In patients taking NSAIDs, although the risk of ulcers is reduced by co-prescription of PPIs or switching to COX-2 inhibitors[35]
, the risk is not abolished and, where possible, alternative analgesia should be used. COX-2 inhibitors are also associated with an increase in cardiac risk and are contraindicated in patients with established heart or cerebrovascular disease.
Routine follow-up endoscopy should be arranged at 4–6 weeks to confirm gastric ulcer healing; however this is unnecessary for duodenal ulcers.
Variceal bleeding
There is no clear role for PPIs after a variceal bleed. A RCT of pantoprazole showed a reduction in size of banding induced ulcers; however, there was no difference in the number of ulcers or patient symptoms[36]
.
Non-selective beta-blockers should be started for secondary prevention of variceal bleeding. Propranolol (starting dose 40mg twice daily) has the strongest evidence for secondary prevention, as it reduces portal pressures through splanchnic vasoconstriction and reduced cardiac output. In a meta-analysis of 12 trials (11 using propranolol), beta blockers increased freedom from rebleeding (21% mean improvement rate; 95% CI: 10–32%,) and increased survival (5.4%; 95% CI: 0–11%), and the mean percentage of patients free of bleeding death (7.4%; 95% CI: 2–13%)[37]
. An alternative is carvedilol (6.25–12.50mg once daily), which has been shown to achieve a greater reduction in portal pressure than propranolol. Pharmacological therapy should be titrated to a maximum tolerated dose aiming for a resting heart of rate of 50–55 beats per minute, and used in combination with repeated variceal banding to achieve eradication of varices[38]
.
Endoscopy should be repeated 2–4 weeks after variceal haemorrhage for repeat banding to ensure eradication of varices.
Summary
AUGIB is a common medical emergency. A combination of both endoscopic and pharmacological treatments play an important role in reducing both mortality and morbidity arising from peptic ulcer and variceal bleeding, which are the most common underlying causes.
Financial and conflicts of interest disclosure:
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was used in the production of this manuscript.
References
[1] Button L, Roberts S, Evans PA et al. Hospitalized incidence and case fatality for upper gastrointestinal bleeding from 1999 to 2007. Aliment Pharmacol Ther 2011;33: 64–76. doi: 10.1111/j.1365-2036.2010.04495.x
[2] Hearnshaw SA, Logan RF, Lowe D et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011;60:1327–1335. doi: 10.1136/gut.2010.228437
[3] Crooks CJ, Card TR & West J. Trends in mortality of non-variceal upper gastrointestinal haemorrhage in England: analysis of hospital admissions 1999 to 2005. Gut 2009;558 (Suppl 11):A93.
[4] Hernandez-Diaz S & Rodriguez LA. Association between non steroidal anti-inflammatory drugs and upper gastro intestinal tract bleeding/perforation: An overview of epidemiologic studies published in the 1990s. Arch Intern Med 2000;160(14):2093–2099. doi: 10.1001/archinte.160.14.2093
[5] Lanza FL, Chan FK & Quigley EM. Practice Parameters Committee of the American College of Gastroenterology 2009 Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 2009;104:728–738. doi: 10.1038/ajg.2009.115
[6] GarcÃa RodrÃguez LA & Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994;343:769–772. doi: 10.1016/S0140-6736(94)91843-0
[7] Garcıa Rodrıguez LA, Lin J K, Hernandez-Dıaz S & Johansson S. Risk of upper gastrointestinal bleeding with low-dose acetylsalicylic acid alone and in combination with clopidogrel and other medications. Circulation 2011; 123:1108–1115. doi: 10.1161/CIRCULATIONAHA.110.973008
[8] Jiang HY, Chem HZ, Hu XJ et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2015;13:42–50. doi: 10.1016/j.cgh.2014.06.021
[9] National Confidential Enquiry into Patient Outcome and Death. Time to get control? A review of the care received by patients who had a severe gastrointestinal haemorrhage. 2015 Available at: https://www.ncepod.org.uk/2015gih.html (accessed October 2018)
[10] National Institute for Health and Care Excellence. Acute upper gastrointestinal bleeding in over 16s: management. 2016. Available at: www.nice.org.uk/guidance/cg141 (accessed October 2018)
[11] Blatchford O, Murray WR & Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 2000;356:1318–1321. doi: 10.1016/S0140-6736(00)02816-6
[12] Rockall TA, Logan RF, Devlin HB et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996;38:316–321. doi: 10.1136/gut.38.3.316
[13] Villanueva C, Colomo A, Bosch A et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21. doi: 10.1056/NEJMoa1211801
[14] Jairath V, Kahan B, Gray A et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet 2015;386:137–144. doi: 10.1016/S0140-6736(14)61999-1
[15] Gralnek IM, Dumonceau JM, Kuipers EJ et al. Diagnosis and management of nonvariceal uppergastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:1–46. doi: 10.1055/s-0034-1393172
[16] Keeling D, Baglin T, Tait C et al. Guidelines on oral anticoagulation with warfarin – 4th ed. Br J Haematol 2011;154(3):311–324. doi: 10.1111/j.1365-2141.2011.08753.x
[17] Tomaselli FG, Mahaffey KW, Cuker A et al. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants. J Am Coll Cardiol 2017;70(24):3042–3067. doi: 10.1016/j.jacc.2017.09.1085
[18] Sreedharan A, Martin J, Leontiadis GI et al. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev 2010;7:CD005415. doi: 10.1002/14651858.CD005415.pub3
[19] Theivanayagam S, Lim RG, Cobell WJ et al. Administration of erythromycin before endoscopy in upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. Saudi J Gastroenterol 2013;19:205–210. doi: 10.4103/1319-3767.118120
[20] Bennett C, Klingenberg SL, Langholz E & Gluud LL. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst Rev 2014;11:CD006640. doi: 10.1002/14651858.CD006640.pub3
[21] Haemorrhage Alleviation With Tranexamic Acid- Intestinal System (HALT-IT) trial. (NCT01658124). Available at: https://clinicaltrials.gov/ct2/show/NCT01658124 (accessed October 2018)
[22] Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI et al. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. Cochrane Database Syst Rev 2010;9: CD002907. doi: 10.1002/14651858.CD002907.pub2
[23] Ioannou GN, Doust J & Rockey DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev 2003;1: CD002147. doi: 10.1002/14651858.CD002147
[24] Calvet X, Vergara M, Brullet E et al. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high risk bleeding ulcers. Gastroenterology 2004;126:441–450. doi: 10.1053/j.gastro.2003.11.006
[25] Laine L & Cooke D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding. Ann Intern Med 1995;123:280–287. doi: 10.7326/0003-4819-123-4-199508150-00007
[26] Jairath V, Kahan B, Logan R et al. National audit of the use of surgery and radiological embolization after failed endoscopic haemostasis for non-variceal upper gastrointestinal bleeding. Br J Surg 2012;99:1672–1680. doi: 10.1002/bjs.8932
[27] Leontiadis GI, Sharma VK & Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev 2010;5:CD002094. PMID: 15266462
[28] Barkun AN, Bardou M, Kuipers EJet al. International consensus recommendations on the management of patients with nonvariceal upper gastro-intestinal bleeding. Ann Intern Med 2010;152:101–113. doi: 10.7326/0003-4819-152-2-201001190-00009
[29] Sachar H, Vaidya K & Laine L. Intermittent vs continuous proton pump inhibitor therapy for high-risk bleeding ulcers: a systematic review and meta-analysis. JAMA Intern Med 2014;174:1755–1762. doi: 10.1001/jamainternmed.2014.4056
[30] Gisbert JP, Khorrami S, Carballo F et al. Helicobacter pylori eradication therapy vs. antisecretory non-eradication therapy (with or without long-term maintenance antisecretory therapy) for the prevention of recurrent bleeding from peptic ulcer. Cochrane Database Syst Rev 2004;2:CD004062. doi: 10.1002/14651858.CD004062.pub2
[31] Scottish Intercollegiate Guidelines Network. Management of Acute Upper and Lower Gastro-intestinal Bleeding Guideline 105. 2008. Available at: https://www.sign.ac.uk/sign-105-management-of-acute-upper-and-lower-gastrointestinal-bleeding.html (accessed October 2018)
[32] Sung JJ, Lau JY, Ching JY et al. 2010 Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med 2010;152:(1)1–9. doi: 10.7326/0003-4819-152-1-201001050-00179
[33] Lai KC, Lam SK, Chu KM et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low dose aspirin use. N Engl J Med 2002;346:2033–2038. doi: 10.1056/NEJMoa012877
[34] Medicines and Healthcare products Regulatory Agency. Clopidogrel and proton pump inhibitors: interaction—updated advice. Drug Safety Update 2010;3(9): 4. Available at: https://www.gov.uk/drug-safety-update/clopidogrel-and-proton-pump-inhibitors-interaction-updated-advice (accessed October 2018)
[35] Hooper L, Brown TJ, Elliott RA et al. The effectiveness of five strategies for the prevention of gastro-intestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. BMJ 2004;329:948–952. doi: 10.1136/bmj.38232.680567.EB
[36] Shaheen NJ, Stuart E, Schmitz SM et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology 2005;41:588–594. doi: 10.1002/hep.20593
[37] Bernard B, Lebrec D, Mathurin P et al. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Hepatology 1997;25:63–70. doi: 10.1053/jhep.1997.v25.pm0008985266
[38] Tripathi D, Stanley AJ, Hayes PC et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680–1704. doi: 10.1136/gutjnl-2015-309262


