This content was published in 2009. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Objectives
After studying this article, you should be able to:
- List five medicines that can cause hyperkalaemia and the mechanism by which they do so
- Define the role of calcium salts in the treatment of hyperkalaemia
- Describe the mechanism of action and place in therapy for three treatments of hyperkalaemia
Potassium is the most abundant cation in the body. Of the 4,000mmol present in the average person, about 98% is intracellular. Dietary potassium is readily absorbed and short-term homeostasis is maintained by insulin and catecholamines. The main role of potassium is to maintain cell membrane potential — important for neuronal transmission and skeletal and cardiac muscle contraction. Patients who develop hyperkalaemia are at high risk of death, especially those in acute renal failure. The severity of hyperkalaemia is classified according to serum potassium levels:
- Mild — 5.5–6mmol/L
- Moderate — 6.1–6.9mmol/L
- Severe — ≥7mmol/L
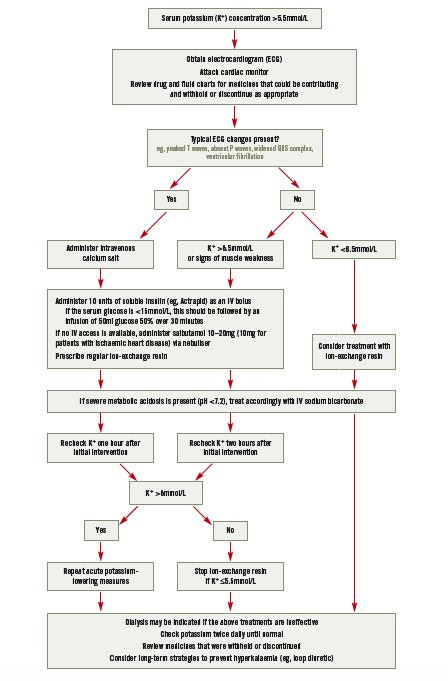
Hyperkalaemia is often asymptomatic and detected only by routine laboratory tests. Its early symptoms are generally non-specific and include malaise, vague muscle weakness and paraesthesia. Certain electrocardiogram (ECG) changes may suggest hyperkalaemia and, although these correlate poorly with potassium concentration, are a guide to the impact of the electrolyte imbalance on the patient — see treatment algorithm attached to the PDF of this article online (www.pjonline.com/learning#light).
Non-drug causes
Normally, the kidneys are responsible for excreting 90–95% of the average daily potassium load (about 100mmol), so renal failure can often cause hyperkalaemia. Damage to body tissues (eg, caused by trauma, burns, rhabdomyolysis or tumour lysis) can release sufficient intracellular potassium to cause hyperkalaemia.
In response to acidosis, cells in the human body compensate by exchanging intracellular potassium for extracellular protons. For every 0.1 unit decrease in blood pH, serum potassium increases by about 0.6mmol/L (although this can be less if the acidosis is caused by organic acids1).
Aldosterone increases potassium secretion in the distal nephron. Therefore, Addison’s disease and hyporeninaemic hypoaldosteronism (a rare complication of long-standing diabetes and renal failure) are predictable causes of hyperkalaemia.
It is important to be aware that pseudohyperkalaemia can sometimes occur. This is when the measured potassium concentration is artificially elevated by an in vitro release of potassium from blood cells. It may occur as a result of mechanical trauma during venepuncture or excessive agitation or poor storage of blood samples; in such cases another sample should be taken. It can also occur if the patient suffers from severe leucocytosis or thrombocytosis.
Drug causes
There are three mechanisms by which medicines can cause hyperkalaemia.
Increased potassium intake
Since the total extracellular potassium store is only about 80mmol, rapid administration of intravenous potassium can cause hyperkalaemia. Other exogenous sources include oral potassium supplements, salt substitutes (which often contain potassium chloride) and medicines formulated as potassium salts.
Decreased excretion
Medicines that interfere with the secretion and action of aldosterone can cause hyperkalaemia. Renin, secreted in response to reduced renal perfusion, activates the angiotensinaldosterone system. Its secretion is mediated by prostaglandins. The following medicines can interfere with this process:
- Non-steroidal anti-inflammatory drugs — inhibit prostaglandin production
- Angiotensin-converting enzyme inhibitors and angiotensin-II receptor antagonists — impair angiotensin IImediated secretion of aldosterone
- Spironolactone and eplerenone — inhibit the binding of aldosterone to receptors
- Heparin — aldosterone synthesis is inhibited by both unfractionated and low molecular weight heparins (the effect being maximal after 3–5 days)
Calcineurin inhibitor immunosuppressants (eg, ciclosporin, tacrolimus) can cause hyperkalaemia. Also, trimethoprim, particularly when prescribed at the high doses used to treat Pneumocystis jiroveci pneumonia, and the potassiumsparing diuretics amiloride and triamterene reduce potassium secretion in the kidney.
Altered cellular distribution
The transmembrane Na/K-ATPase pump, which maintains the ratio of intracellularto- extracellular potassium concentrations, is inhibited by digoxin. This effect is small at therapeutic concentrations, but significant if digoxin reaches toxic levels.
Beta-blockers inhibit the catecholamineinduced entry of potassium into cells, although in practice the resulting increase in potassium concentration is generally less than 0.5mmol/L. Suxamethonium opens ion channels, thereby causing movement of potassium out of cells.
Discussion points
- How does your local practice differ from that suggested in this article?
- What monitoring would you recommend for a patient being treated for hyperkalaemia?
- At your hospital, are calcium salts used cautiously in patients taking digoxin concurrently?
Management
Medicines that might cause potassium accumulation should be stopped or withheld where appropriate. For mild hyperkalaemia, the addition of a loop or thiazide diuretic, to enhance renal excretion of potassium, and treatment with an ionexchange resin may be sufficient. Emergency treatment of hyperkalaemia is needed if serum potassium is greater than 6.5mmol/L or if a patient with mild or moderate hyperkalaemia experiences ECG changes or symptoms of muscle weakness.
Calcium salts
Calcium salts do not affect serum potassium concentration but can reduce the risk of arrhythmias by antagonising cardiac membrane excitability; therefore, they should be administered to patients who experience ECG changes characteristic of hyperkalaemia. In practice, a single IV dose of a calcium salt is often given prophylactically in the absence of ECG changes.
IV calcium gluconate or calcium chloride can be used, although the former is preferred because it is less of an irritant. Either 10ml of calcium gluconate 10% or 5ml of calcium chloride 10% should be administered over two minutes. If there is no ECG improvement within 10 minutes of administration, the dose should be repeated until the ECG normalises. The effect lasts 30–60 minutes so repeat doses might be needed.
Despite a paucity of information to suggest a clinical problem, it has been suggested that transient hypercalcaemia occurring after calcium salts have been administered rapidly may potentiate the myocardial toxicity of digoxin.2 Current guidelines recommend that for those taking digoxin the dose of calcium salt should be infused over 20 minutes.3 In an emergency, this advice is rarely followed and, anecdotally, there does not appear to be any excess mortality.
Insulin
Insulin activates the Na/K-ATPase responsible for moving potassium into cells. A mixture of 10u of soluble insulin and 50ml of glucose 50% is administered by IV injection over 15–30 minutes. Patients with a serum glucose above 15mmol/L can be given insulin alone.2,3 Due to theoretical concerns that insulin may be denatured by high concentrations of glucose, it may be preferable to administer insulin as an IV bolus immediately before starting a glucose infusion. Blood glucose should be monitored 30 minutes after the infusion finishes and hourly for a further six hours. A reduction in potassium concentration of 0.5–1mmol/L can be expected within 15 minutes and persists for four to six hours.3
Salbutamol
Stimulation of beta2 adrenergic receptors also enhances cellular uptake of potassium by muscle and liver cells via activation of the Na/K-ATPase, but independent of the action of insulin. IV and nebulised salbutamol are similarly effective, reducing potassium concentration by a maximum of about 1mmol/L and lasting for at least two hours.2,3
Resistance to the potassium-lowering effect of salbutamol is relatively common so it is not recommended for use as a single agent. Combination of salbutamol with insulin has been shown to be more effective than either intervention alone.4
Sodium bicarbonate
Sodium bicarbonate is no longer recommended as routine treatment for hyperkalaemia. It may be used for hyperkalaemia associated with severe metabolic acidosis (pH <7.2). Flushing of lines between calcium salt and sodium bicarbonate administration is essential to prevent precipitation.
Potassium removal
Moving potassium ions into cells does not obviate the need to remove excess potassium from the body. Since the excretion of potassium depends largely on urine flow, loop diuretics can increase potassium excretion — provided renal function is adequate.
Ion-exchange resins, such as calcium polystyrene sulphonate, bind potassium in the gastrointestinal tract and increase faecal elimination. They must reach the colon to be effective so have a delayed onset of action (>4 hours). They are often prescribed with an osmotic laxative (eg, lactulose) to prevent constipation.
Regular monitoring of potassium concentration (ie, twice daily) is essential and ion-exchange resins should not be given if the concentration is below the upper limit of normal (as specified in local guidelines). This reduces the risk of hypokalaemia and is necessary because their action persists for many hours after administration.
Renal replacement therapy (ie, haemodialysis or haemofiltration) is the most effective and rapid mechanism for lowering potassium levels and total body potassium. However, it usually requires significant preparation and is generally reserved for those with severe renal impairment or for whom first-line treatments have failed.
References
- Rastergar A, Soleimani M. Hypokalaemia and hyperkalaemia. Postgraduate Medical Journal 2001;77:759–64.
- Kim H, Han S. Therapeutic approach to hyperkalemia. Nephron 2002;92(s1):33–40.
- Clinical Resource Efficiency Support Team. Guidelines for the treatment of hyperkalaemia in adults. 2006. www.crestni.org.uk (accessed 23 July 2009).
- Ahee P, Crowe AV. The management of hyperkalaemia in the emergency department. Journal of Accident & Emergency Medicine 2000;17:188–91.
Hyperkalaemia treatment algorithm. For adults in non-critical areas