Shutterstock.com
Nephrotic syndrome (NS) is a rare, serious and debilitating kidney condition, caused by a range of different diseases that damage the glomeruli. The condition affects around 1 in 30,000 UK adults per year[1].
Glomeruli are found in the kidneys and filter fluid, electrolytes and waste products from the blood, while preventing the loss of protein, higher molecular weight solutes and cellular compartments[2]. Damage to the glomeruli allows proteins, such as albumin, to pass through into the nephron tubules and be lost in urine, giving rise to three clinical features that define NS:
- Proteinuria (defined as loss of >3.5g of protein in urine over 24 hours);
- Hypoalbuminaemia (<30g/L of albumin in the blood);
- Peripheral oedema[3].
There are several associated complications in NS, some of which result from compensatory overproduction of proteins by the liver. Common complications include high levels of lipids in the blood (hyperlipidaemia), thromboembolism and an increased risk of infection[3].
Pathological injury to the renal glomeruli may be caused by a primary disease or secondary to a systemic disorder (e.g. diabetes)[3].
Although a rare condition affecting adults and children, NS is an important manifestation of kidney disease accompanied by several different clinical features requiring pharmacological management; therefore, it is important that pharmacists in all sectors are aware of the signs and symptoms, possible complications and evidence-based management.
Pathophysiology
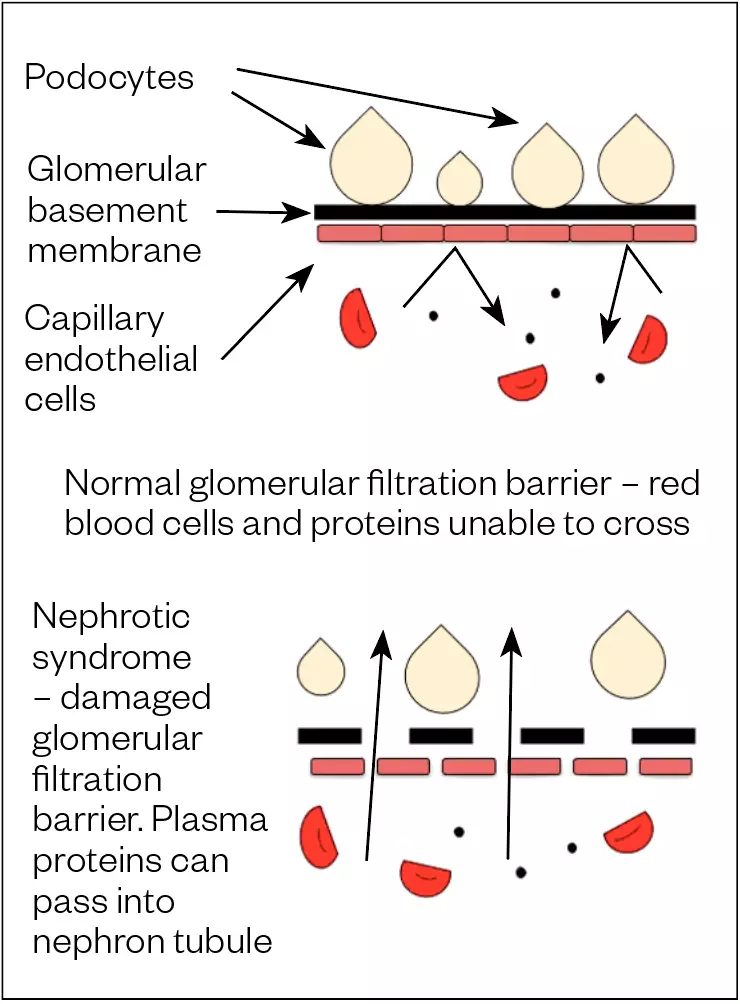
The glomerulus is the site of blood filtration within the kidneys where fluid and other substances pass from the capillaries into the nephron[2]. This process is guarded by the glomerular filtration barrier (GFB), a membrane that restricts the passage of serum proteins by size and charge. It consists of three layers:
- Capillary endothelial cells;
- Glomerular basement membrane (GBM);
- Epithelial cells (podocytes)[4].
In NS, injuries to the GFB increase permeability to serum proteins through changes in its charge-selective or size-selective properties, causing proteinuria[5]. One or more changes, including damage to the podocytes, capillary endothelial cells and disruption of the GBM function (see Figure 1) may be seen in any one type of NS[5].
Injury to podocytes — the major target of injury in diseases causing primary NS — is commonly caused by immune complex deposition or complement protein activation[5].
Complications
In response to increased glomerular permeability to large molecules (e.g. albumin), the liver tries to compensate for this protein loss by increasing the production of albumin, as well as other molecules, including low-density lipoproteins, giving rise to lipid abnormalities (e.g. hypercholesterolaemia and hypertriglyceridemia)[6,7].
Oedema (i.e. swelling in the body owing to fluid accumulation) is the major clinical manifestation of NS. It is caused by a decrease in plasma oncotic pressure from the urinary loss of albumin, which promotes the movement of fluid from the vascular space into the interstitium. This leads to underfilling of the vasculature and secondary sodium retention through the activation of the renin-angiotensin-aldosterone system (RAAS). Additionally, there is primary sodium retention in the renal tubules induced by the renal disease[8,9].
People with NS are at an increased risk of venous thromboembolism (VTE), which — in some instances — may be the presenting complaint. This arises from hypercoagulability thought to be owing to urinary anticoagulant protein losses and increased synthesis of coagulation factors by the liver[10,11]. An individual’s risk of infection also increases owing to the loss of immunoglobulins (antibodies) in the urine[3].
Symptoms
Progressive oedema, mainly observed in the lower limbs (see Figure 2), periorbital (around the eyes), genital area and abdomen (ascites; see Figure 3), is the typical presenting symptom of NS[12]. Urine may appear frothy owing to high concentrations of protein (see Figure 4)[12]. Fatigue and loss of appetite are also common symptoms, with others attributable to the complications of NS (see Table 1)[10,12–14].
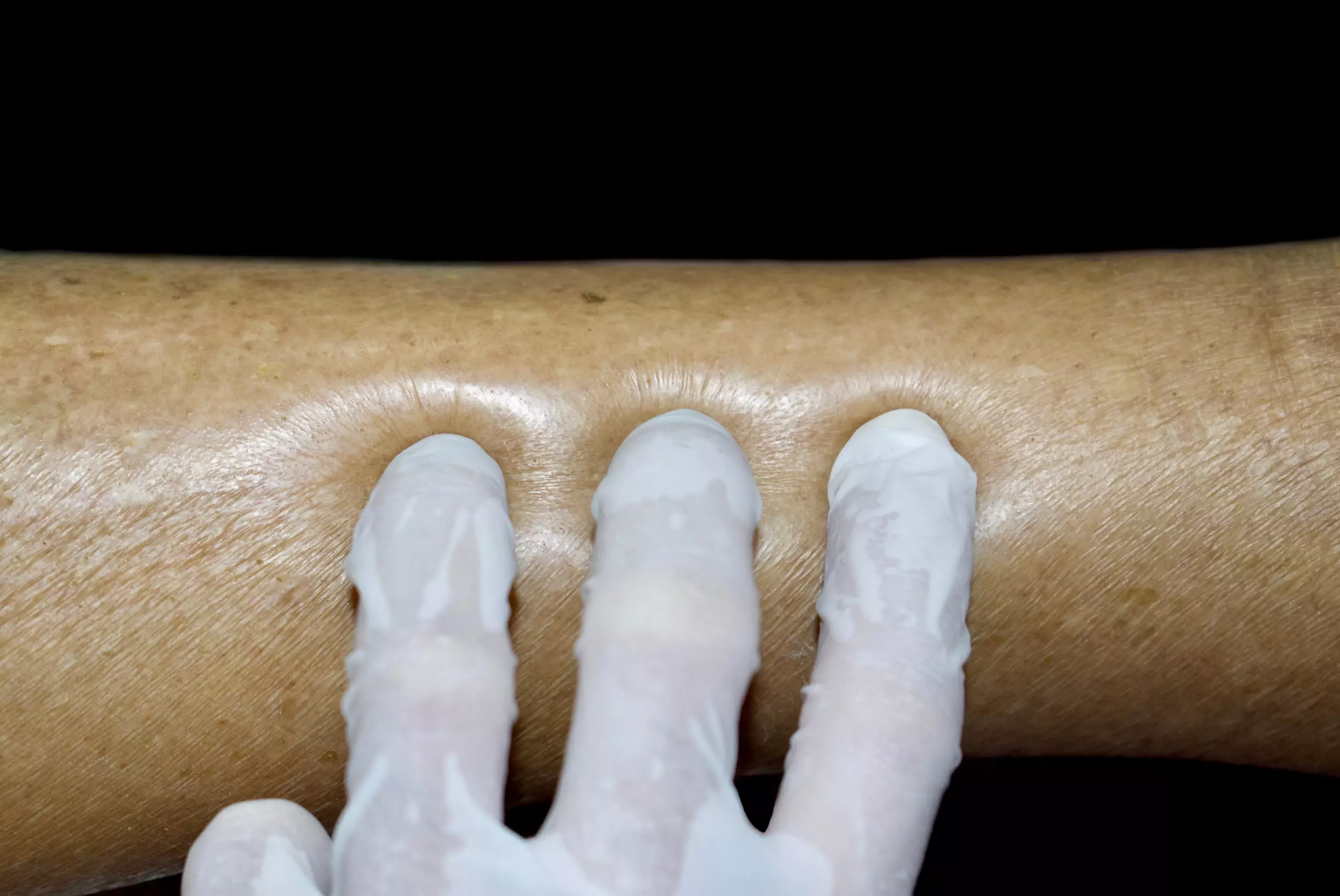
Shutterstock.com
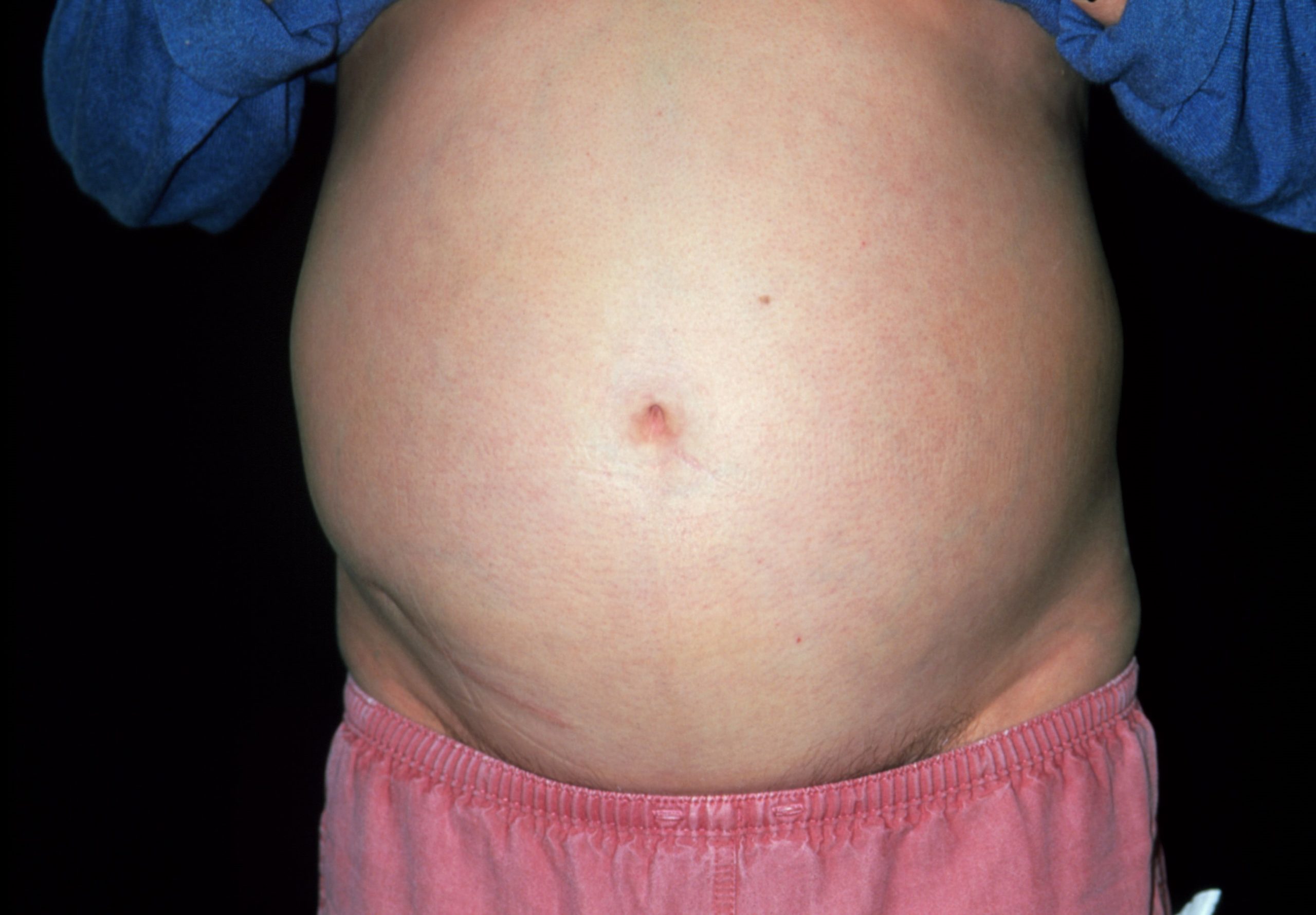
Science Photo Library
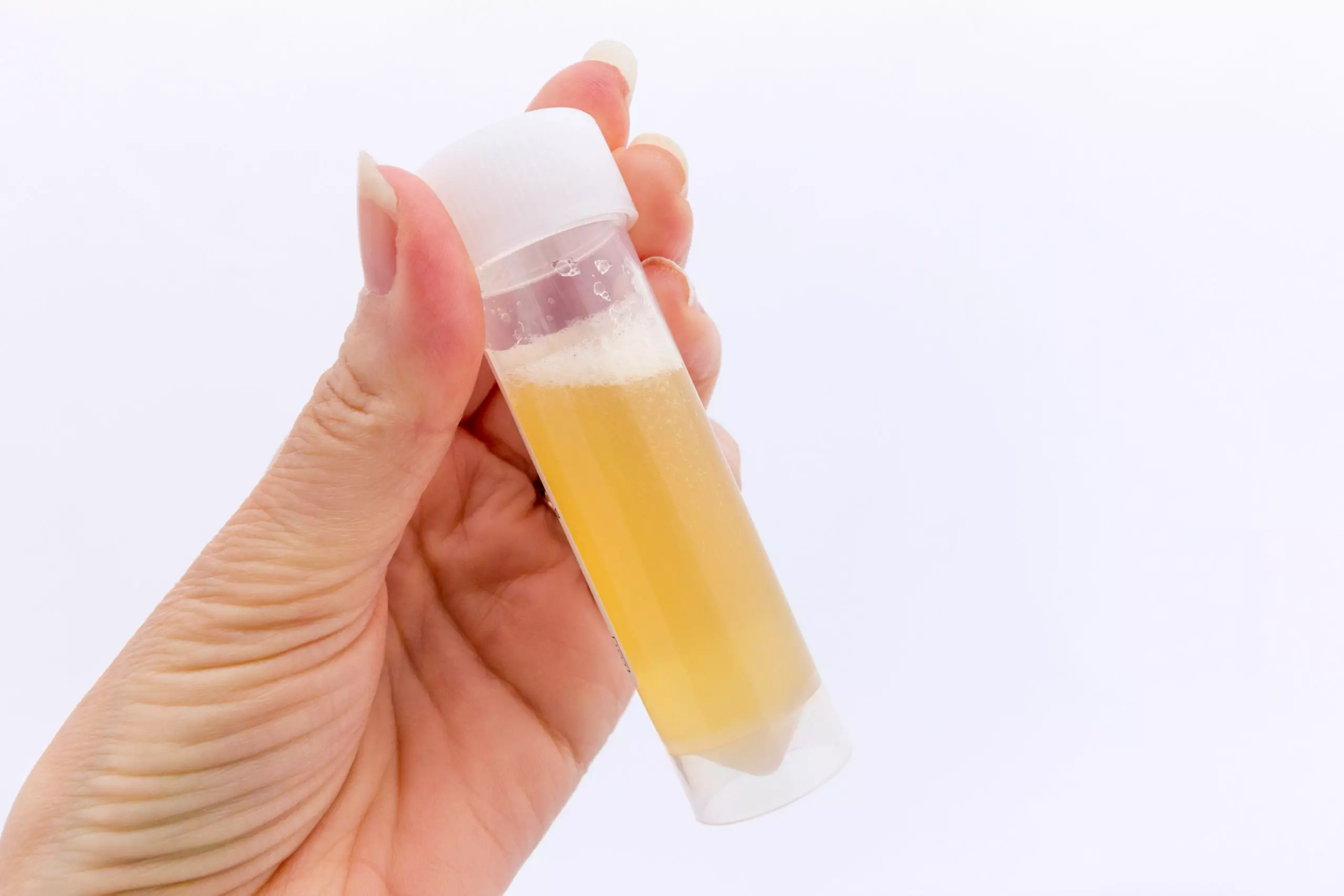
Shutterstock.com
Diagnosis may be complicated by the presence of symptoms that are more commonly encountered in other conditions; for example, peripheral oedema is seen in congestive heart failure and hypoalbuminemia can be caused by severe liver disease[14].
Table 1: Complications of nephrotic syndrome and contributing factors
| Complications | Contributing factors |
| Weight gain | Secondary to fluid retention |
| Dyslipidaemia | Increased hepatic lipoprotein synthesis |
| Hypothyroidism | Loss of thyroid-binding globulin |
| Infection | Loss of circulating immunoglobulins and complement factors |
| Thromboembolic event | Hypercoagulopathy that may be owing to urinary anticoagulant protein losses (e.g. antithrombin, protein C and protein S) and the increased hepatic synthesis of procoagulant factors (e.g. fibrinogen, factor V and factor VIII) |
| Breathlessness | May be owing to pulmonary oedema, pleural effusions or a pulmonary embolism |
| Bone disorders | Corticosteroid use; vitamin D deficiency owing to loss of vitamin D binding protein |
| Hypertension | Owing to renal sodium retention |
| Renal failure | May be related to severe active disease or chronic progressive disease process, but can also occur with the use of diuretics if hypovolaemia occurs |
Causes and risk factors
Causes of NS are classified into either primary or secondary diseases (see Table 2)[12–14].
Primary diseases are described by their histology following a renal biopsy and are usually the result of glomerular injury from a kidney disease such as:
- Focal segmental glomerulosclerosis (FSGS);
- Membranous nephropathy (MN);
- Minimal change disease (MCD)[15,16].
In adults, FSGS accounts for between 35% and 50% of nephrotic causes. FSGS is not a single disease, but rather a pattern of kidney injury characterised by segmental areas of mesangial collapse and sclerosis in some glomeruli[5]. The cause is usually idiopathic but may be secondary (e.g. drug use, HIV and obesity)[5].
MN is one of the most common causes of NS in adults and may be owing to primary or secondary causes (e.g. infections, drugs, autoimmune disorders and cancer). Among adult patients with primary MN, 70–80% of patients have circulating antibodies to the M-type receptor of the anti-phospholipase A2 receptor (anti-PLA2R) located on the podocyte membrane. Measuring these antibodies can help distinguish primary MN[17].
MCD is so called because light microscopy on histology often reveals normal glomeruli and is the most common cause of NS in children, accounting for 90% of cases, although it can also occur in adults[5].
Secondary causes include underlying systemic conditions that affect other organs, in addition to the kidneys. Around 30% of adults with NS have a systemic disease (e.g. diabetes mellitus, amyloidosis, or systemic lupus erythematosus). Diabetic nephropathy is the most common multisystem disease causing NS in adults[5,18].
Table 2: Causes of nephrotic syndrome classified into either primary or secondary diseases
| Common primary causes | Common secondary causes |
| Focal segmental glomerulosclerosis | Amyloidosis |
| Membranous nephropathy | Cancer |
| Membranoproliferative glomerular disease | Diabetes mellitus |
| Minimal change disease | Medicines (e.g. non-steroidal anti-inflammatory drugs, interferons, lithium, intravenous bisphosphonates) |
| Systemic lupus erythematosus | |
| Viral infections; HIV, hepatitis B, hepatitis C |
Diagnosis
Confirmation of proteinuria is initially carried out by a dipstick urinalysis. The amount of protein is quantified by either a 24-hour urine collection or a spot urine protein-to-creatinine ratio (uPCR) with >3.5g/24 hours or uPCR>300mg/mmol indicative of nephrotic-range proteinuria[19].
Once a clinical diagnosis is confirmed, further diagnostic assessment is aimed at identifying the underlying cause and assessing for complications. For example:
- Basic blood biochemistries:
- Urea and creatinine to assess renal excretory function;
- Total cholesterol to detect severe hyperlipidaemia (e.g. >10 mmol/L);
- Serum albumin for thromboembolic risk;
- Thyroid function tests for hypothyroidism;
- Monitoring vitamin D and calcium levels for bone disorders[19];
- Autoimmune screen (undertaken if an autoimmune disease, such as systemic lupus erythematosus, is suspected);
- Virology screen (undertaken to determine whether HIV or hepatitis B or C are causes);
- HbA1c and fasting glucose – to exclude diabetes mellitus as a cause;
- Serum free light chains or urine protein electrophoresis – may suggest amyloidosis or multiple myleloma;
- Renal ultrasound to assess renal size and morphology;
- Renal biopsy to determine histologic type, including immunofluorescence and electron microscopy[20].
If NS is supsected, all patients should be referred to a nephrologist for further investigation and management[12].
Management
Treatment of NS involves two phases: induction of remission followed by maintaining remission to prevent relapse, defined as per the Kidney Disease Improving Global Outcomes guidelines (see Table 3)[21,22].
Immunosuppressive medications may be used to treat some of these primary causes, with the regime dependent on the patient’s diagnosis and response[21,22]. All of these treatments require initiation under the direction of a consultant nephrologist.
Table 3: Nephrotic syndrome treatment response definitions
| Complete remission | Proteinuria reduced to <0.3g/day or uPCR <300mg/g (<30mg/mmol) with stable plasma creatinine and albumin >35g/L |
| Partial remission | Proteinuria reduced to 0.3–<3.5g/day or uPCR 300–<3500mg/g (30–350mg/mmol) and a reduction of >50% from baseline |
| Relapse | Proteinuria >3.5g/day or uPCR >3500mg/g (350mg/mmol) after complete remission previously achieved |
Source: KDIGO[21,22]
Membranous nephropathy
Patients with MN will receive treatment if they are at risk of disease progression. Criteria for the risk of progression consists of:
- High PLA2R antibody levels and heavy proteinuria (>8g/day) that is persistent over six months;
- Severe NS;
- Estimated glomerular filtration rate (eGFR) of <60mL/min/1.73m[20].
If a patient has severe NS with complications or rapidly deteriorating renal function, recommended treatment includes cyclophosphamide and steroids for six months[21,22].
For people with other risk factors for progression, treatment includes steroids plus either cyclophosphamide or rituximab, or calcineurin inhibitor with addition of rituximab for six months[21,22].
Measuring anti-PLA2R levels at three and six months may be useful in evaluating treatment response and decisions about further treatments[21,22].
Dosing regimens include:
- Cyclical cyclophosphamide: intravenous methylprednisolone 1g on days 1 to 3 of months 1, 3 and 5. This is followed by prednisolone 0.5mg/kg daily in months 1, 3 and 5. Cyclophosphamide is given orally (2.5mg/kg) daily in months 2, 4 and 6 (to complete 6 months);
- The maximum cumulative dose of cyclophosphamide should be 25g to minimise long-term risks, including malignancy;
- Intravenous rituximab given as a 1g/dose for 2 doses (2 weeks apart) or 375mg/m2 weekly for 4 doses;
- Oral calcineurin inhibitors in 2 divided doses 12 hours apart (adjusted according to trough levels): ciclosporin (target level: 125–175ng/mL) 3.5mg/kg/day; or tacrolimus (target level: 3–8mg/mL) 0.05-0.1mg/kg/day[21–25].
In clinical practice, intravenous (IV) pulsed cyclophosphamide may be used owing to a lower cumulative dose. A study by Kanigicherla et al., using a regimen of IV cyclophosphamide 600mg/m2 monthly for 6 months with oral prednisolone 0.75mg/kg/day gradually tapered over 6 months, demonstrated remission at a significantly reduced cumulative cyclophosphamide dose[26].
The recent MENTOR trial studied rituximab versus ciclosporin in patients with MN and an eGFR >40mL/min/1.73m2 and proteinuria >5g/day to assess complete and partial remission[24]. At 12 months, there was no significant difference in complete or partial remission between the groups; however, at 24 months, rituximab was superior in terms of maintaining proteinuria remission[24]. Limited data is available on the use of rituximab in treatment-resistant MN but, in 2018, NHS England commissioned rituximab through an evaluation programme to gather data — follow up for this is ongoing[25].
Minimal change disease and primary focal segmental glomerulosclerosis
High-dose oral prednisolone is usually initiated at 1mg/kg/day (up to a maximum of 80mg/day) for around 4–16 weeks, depending on when remission is achieved. Once remission is achieved, prednisolone should be gradually tapered over 6 months[21,22]. MCD is more steroid responsive than other causes of NS, with 80% of cases responding to steroids compared to 20% with FSGS[21,27]. In practice, duration varies according to response.
Several adverse effects associated with the use of high-dose steroids must be monitored and carefully managed (see Table 4)[21,28–30].
Table 4: Management of potential adverse effects from high-dose steroid treatment
| Adverse effects | Management strategy |
| Pneumocystis pneumonia (PCP) | PCP prophylaxis with co-trimoxazole (i.e. sulfamethoxazole and trimethoprim) 480mg daily or 960mg three times per week; If intolerant or allergic to either component of co-trimoxazole, dapsone 50–100mg daily can be initiated (dose dependent on renal function). |
| Fungal infections | Fungal prophylaxis with fluconazole 50mg daily. |
| Risk of gastric ulceration | Advise patients to take prednisolone with food; Gastroprotection with a proton pump inhibitor or H2 receptor antagonist. |
| Osteoporosis risk | Calcium/vitamin D combination supplementationHigh-risk patients (e.g. older people or postmenopausal females) should also receive an appropriate bisphosphate (extra consideration of risk/benefit if estimated glomerular filtration rate<30mL/min/1.73m). |
| Hyperglycaemia | Pre-meal capillary blood glucose (CBG) monitoring;Diabetes specialist nurse referral if significant variations;If CBG>12mmol/L on 2 occasions in 24 hours, gliclazide, or intermediate-acting insulin (Humulin I/Insulatard/Insuman Basal) may be considered;HbA1C;Optimise and titrate doses to maintain CBG’s between 6–10mmol/L. |
In frequently-relapsing or corticosteroid-dependent MCD, the following are the next potential treatment options (see Figure 5):
- Oral cyclophosphamide 2–2.5mg/kg/day for 8 weeks;
- IV rituximab (dosing as MN);
- Oral calcineurin inhibitors for one to two years (dosing as MN);
- If the patient is intolerant to all of the above, mycophenolate mofetil 500mg twice daily can be used and weaned after a minimum of one year[21,22].
In corticosteroid-resistant primary FSGS, calcineurin inhibitors are generally used; if contraindicated then rituximab or mycophenolate may be used[21,22].
Figure 5: Treatment algorithm for minimal change disease and focal segmental glomerulosclerosis per Kidney Disease Improving Global Outcomes guidelines
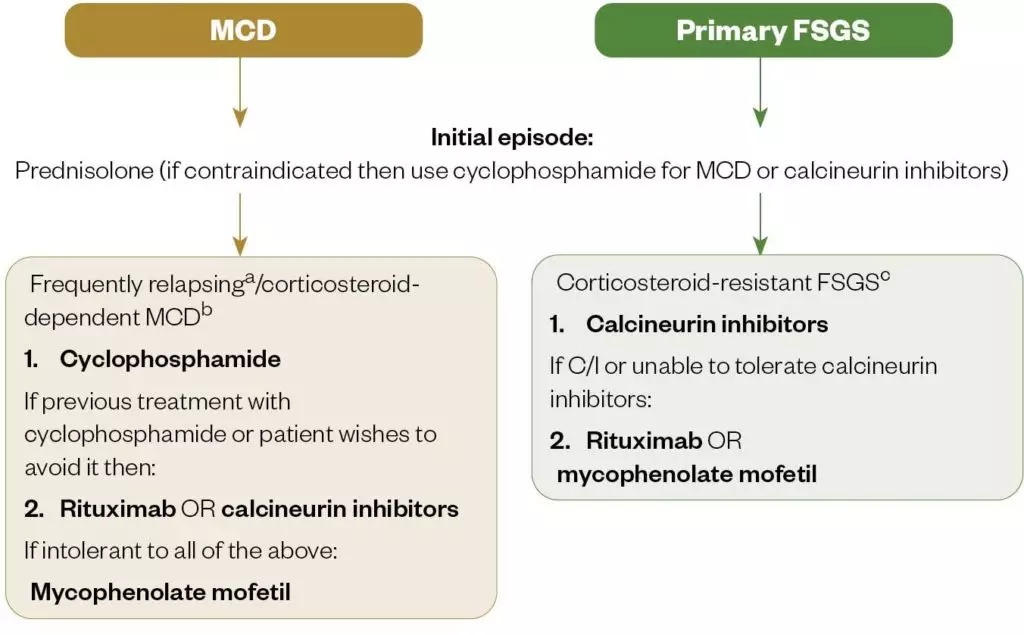
a: frequently relapsing MCD: ≥2 relapses over a 6-month period (or ≥4 over 12 months)
b: corticosteroid-dependent MCD: relapse during or within 2 weeks of prednisolone completion
c: corticosteroid-resistant FSGS: proteinuria persisting >3.5g/day or uPCR >3500 mg/g (350mg/mmol) with <50% reduction from baseline despite prednisolone treatment for at least 16 weeks
Source: Kidney Disease Improving Global Outcomes[21,22]
Based on clinical studies, NHS England have commissioned rituximab for delaying relapses in MCD/FSGS in children; however, it is not commissioned for use in adults owing to a lack of randomised controlled trial data[31]. The TURING study aims to assess the effect of rituximab on time to relapse in patients with MCD/FSGS[32].
In refractory cases of NS plasma exchange, therapy can be attempted[33]. If renal function deteriorates to chronic kidney disease stage 5 (eGFR<15mL/min/1.73m2), then renal replacement therapy in the form of dialysis may be required.
Proteinuria
Angiotensin-converting-enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) can be used to reduce proteinuria[34]. These are often classed as ‘renoprotective’ as they prevent vasoconstriction of the efferent arteriole (which carries blood away from the glomerulus), easing the intraglomerular pressure (hence systemic blood pressure), in turn reducing proteinuria[35]. By inhibiting RAAS, sodium and water retention, exacerbating oedema will also be minimised. These agents are the mainstay of management for patients with diabetes and milder variants of NS.
There are a range of complications associated with nephrotic syndrome and its management, which can be seen in Table 5[21,22,31,34,36–41].
Table 5: Overview of managing complications in a nephrotic patient
| Complications in a nephrotic syndrome | Prevention strategies | Monitoring |
| Proteinuria | Angiotensin-converting-enzyme (ACE) inhibitors (e.g. ramipril, lisinopril) or angiotensin receptor blockers (ARBs) (e.g. losartan, candesartan). | Gradually titrate up to maximum tolerated dose. As an inpatient, titrate per daily blood pressure (BP), and urea and electrolyte (U&E) count. This will be much quicker (every three to five days) owing to closer observation. On discharge, BP and U&Es should be monitored every one to two weeks by the GP, while titrating to the maximum tolerated dose;Risk of hyperkalaemia and acute kidney injury — monitor U&Es as above; Advise patients to monitor for dry, persistent cough from ACE inhibitor (if troublesome, switch to ARB); To minimise first-dose hypotension, take ACE inhibitor at night or split initial dose into twice per day. |
| Oedema | Fluid restriction to 1–1.5L/day; Dietary sodium restriction (<100mmol/day, 2g/day); Loop diuretics daily (e.g. intravenous furosemide 80–120mg/day) initially and titrated as per response. Can step down to oral furosemide or bumetanide. Furosemide requires protein binding to reach its site of action in the renal tubule, which is reduced owing to hypalbuminaemia; therefore, higher doses may be required in resistant cases; Plus or minus: thiazide diuretics, potassium-sparing diuretics or metolazone, which can act synergistically; Plus or minus IV albumin (in resistant cases to aid delivery of furosemide to site of action). | Monitor weight and fluid balance daily as an inpatient (target weight reduction 0.5–1kg/day). This can enable appropriate diuretic dose adjustment. At home, it may be useful for the patient to record a daily weight log and report to the consultant at their outpatient clinic; Risk of hypotension (low blood pressure), hyponatraemia (low sodium), hypokalaemia (low potassium), hypomagnesaemia (low magnesium), alkalosis and acute renal failure if fluid taken off too quickly — close monitoring of daily BP, U&Es and renal function is necessary as an inpatient, and every one to two weeks by the GP on discharge until a stable dose is reached; Advise patients to take diuretics in the morning and at lunch (if twice-daily dose) to minimise sleep disturbance from diuresis. |
| Hyperlipidaemia | Tends to correct itself on treating the cause; If high cardiovascular risk, lipid-lowering therapy, such as statins/ezetimibe can be initiated, although there is limited evidence to suggest efficacy in nephrotic syndrome (NS). | Lipid profile taken on diagnosis and then every three to six months. |
| Hypothyroidism | If thyroid function tests (TFTs) are deranged and patient is symptomatic, option to treat with levothyroxine 50–100mcg daily in the morning. | Test TFTs when NS first diagnosed and annually for those who remain nephrotic. |
| Infection | Prophylactic antibiotics not advised. | Patients advised to receive pneumococcal vaccine every five years and yearly influenza vaccines. |
| Thromboembolism | If nephrotic range proteinuria and serum albumin <25g/dL, anticoagulation may be considered; Warfarin is the preferred agent or low-molecular-weight heparin could be used as an alternative. | Regular international normalised ratio (with warfarin); Consider stopping prophylactic anticoagulation if persistent albumin >25g/dL and no other indication to continue. |
Summary
- Nephrotic syndrome is a collection of signs and symptoms indicating damage to the glomerular filtration barrier that is characterised by proteinuria (> 3.5g/24 hours), hypoalbuminemia and oedema.
- Treatment involves addressing the underlying cause, as well as taking steps to reduce proteinuria, including an angiotensin-converting-enzyme inhibitor, good blood pressure control and management of oedema via sodium and fluid restriction, and the use of diuretics.
- Further management is aimed at assessing and managing the potential complications of nephrotic syndrome, including the risk of thromboembolism, hypercholesterolaemia and infection.
- Nephrotic syndrome related to primary conditions may require treatment with immunosuppressive therapies under specialist nephrology services.
- 1Nephrotic Syndrome in Adults. NeST Nephrotic Syndrome Trust. 2020.https://nstrust.co.uk/who-we-are/nephrotic-syndrome-in-adults (accessed Feb 2021).
- 2Rang H, Ritter J, Flower R, et al. Rang & Dale’s Pharmacology. 8th ed. Edinburgh: : Churchill Livingstone 2016.
- 3The British Medical Journal Best Practice. Assessment of nephrotic syndrome — differential diagnosis of symptoms. 2020.https://bestpractice.bmj.com/topics/en-gb/356/diagnosis-approach (accessed Feb 2021).
- 4What is the anatomy of the glomerular filtration barrier? Medscape. 2020.https://www.medscape.com/answers/244631-154707/what-is-the-anatomy-of-the-glomerular-filtration-barrier (accessed Feb 2021).
- 5Kelepouris E, Rovin B. Overview of heavy proteinuria and the nephrotic syndrome. UpToDate. 2019.https://www.uptodate.com/contents/overview-of-heavy-proteinuria-and-the-nephrotic-syndrome (accessed Feb 2021).
- 6Crew RJ, Radhakrishnan J, Appel G. Complications of the nephrotic syndrome and their treatment. CN 2004;:245–59. doi:10.5414/cnp62245
- 7Wheeler DC, Bernard DB. Lipid Abnormalities in the Nephrotic Syndrome: Causes, Consequences, and Treatment. American Journal of Kidney Diseases 1994;:331–46. doi:10.1016/s0272-6386(12)80994-2
- 8Humphreys MH. Mechanisms and management of nephrotic edema. Kidney International 1994;:266–81. doi:10.1038/ki.1994.33
- 9Perico N, Remuzzi G. Edema of the Nephrotic Syndrome: The Role of the Atrial Peptide System. American Journal of Kidney Diseases 1993;:355–66. doi:10.1016/s0272-6386(12)70137-3
- 10Kauffmann RH, Veltkamp JJ, Van Tilburg NH, et al. Acquired antithrombin III deficiency and thrombosis in the nephrotic syndrome. The American Journal of Medicine 1978;:607–13. doi:10.1016/0002-9343(78)90848-3
- 11Rabelink TJ, Zwaginga JJ, Koomans HA, et al. Thrombosis and hemostasis in renal disease. Kidney International 1994;:287–96. doi:10.1038/ki.1994.274
- 12Hull RP, Goldsmith DJA. Nephrotic syndrome in adults. BMJ 2008;:1185–9. doi:10.1136/bmj.39576.709711.80
- 13Overview of nephrotic syndrome. MSD Manual Professional Edition. 2020.https://www.msdmanuals.com/en-gb/professional/genitourinary-disorders/glomerular-disorders/overview-of-nephrotic-syndrome (accessed Feb 2021).
- 14Kodner C. Diagnosis and Management of Nephrotic Syndrome in Adults. Am Fam Physician 2016;93:479–85.https://www.ncbi.nlm.nih.gov/pubmed/26977832
- 15Haas M, Meehan SM, Karrison TG, et al. Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976–1979 and 1995–1997. American Journal of Kidney Diseases 1997;:621–31. doi:10.1016/s0272-6386(97)90485-6
- 16Orth SR, Ritz E. The Nephrotic Syndrome. N Engl J Med 1998;:1202–11. doi:10.1056/nejm199804233381707
- 17Qin W, Beck LH Jr, Zeng C, et al. Anti-Phospholipase A2 Receptor Antibody in Membranous Nephropathy. JASN 2011;:1137–43. doi:10.1681/asn.2010090967
- 18Diabetic nephropathy. MSD Manual Professional Edition. 2020.https://www.msdmanuals.com/en-gb/professional/genitourinary-disorders/glomerular-disorders/diabetic-nephropathy (accessed Feb 2021).
- 19Methven S, MacGregor MS, Traynor JP, et al. Comparison of Urinary Albumin and Urinary Total Protein as Predictors of Patient Outcomes in CKD. American Journal of Kidney Diseases 2011;:21–8. doi:10.1053/j.ajkd.2010.08.009
- 20Floege J, Barbour SJ, Cattran DC, et al. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney International 2019;:268–80. doi:10.1016/j.kint.2018.10.018
- 21Clinical Guidelines for Glomerulonephritis. Kidney Disease Improving Global Outcomes. 2012.https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf (accessed Feb 2021).
- 22Public review draft of the clinical practice guidelines on glomerular diseases. Kidney Disease Improving Global Outcomes. 2020.https://kdigo.org/wp-content/uploads/2017/02/KDIGO-GN-GL-Public-Review-Draft_1-June-2020.pdf (accessed Feb 2021).
- 23Ponticelli C, Altieri P, Scolari F, et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 1998;9:444–50.https://www.ncbi.nlm.nih.gov/pubmed/9513907
- 24Fervenza FC, Appel GB, Barbour SJ, et al. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N Engl J Med 2019;:36–46. doi:10.1056/nejmoa1814427
- 25Specialised commissioning drugs briefing: spring 2019. NHS England. 2019.https://www.sps.nhs.uk/wp-content/uploads/2019/04/Spec-Comm-Drugs-Briefing-Spring-2019.pdf (accessed Feb 2021).
- 26Kanigicherla DAK, Hamilton P, Czapla K, et al. Intravenous pulse cyclophosphamide and steroids induce immunological and clinical remission in New-incident and relapsing primary membranous nephropathy. Nephrology 2017;:60–8. doi:10.1111/nep.12955
- 27Pérez V, López D, Boixadera E, et al. Comparative differential proteomic analysis of minimal change disease and focal segmental glomerulosclerosis. BMC Nephrol Published Online First: 3 February 2017. doi:10.1186/s12882-017-0452-6
- 28Park JW, Curtis JR, Moon J, et al. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2017;:644–9. doi:10.1136/annrheumdis-2017-211796
- 29Summary of product characteristics for dapsone. Electronic Medicines Compendium. 2019.https://www.medicines.org.uk/emc/product/5769/smpc (accessed Feb 2021).
- 30Management of hyperglycaemia and steroid therapy. Joint British Diabetes Societies for inpatient care. 2014.https://www.diabetes.org.uk/resources-s3/2017-09/JBDS%20management%20of%20hyperglycaemia%20and%20steriod%20therapy_0.pdf (accessed Feb 2021).
- 31Endocrine dysfunction in the nephrotic syndrome. UpToDate. 2020.https://www.uptodate.com/contents/endocrine-dysfunction-in-the-nephrotic-syndrome (accessed Feb 2021).
- 32The use of rituximab in the treatment of nephrotic glomerulonephritis (TURING). ISRCTN Registry. 2019.http://www.isrctn.com/ISRCTN16948923 (accessed Feb 2021).
- 33Vlachopanos G, Georgalis A, Gakiopoulou H. Plasma Exchange for the Recurrence of Primary Focal Segmental Glomerulosclerosis in Adult Renal Transplant Recipients: A Meta-Analysis. Journal of Transplantation 2015;:1–7. doi:10.1155/2015/639628
- 34Kodner C. Nephrotic syndrome in adults: diagnosis and management. Am Fam Physician 2009;80:1129–34.https://www.ncbi.nlm.nih.gov/pubmed/19904897
- 35PRESTON R. Renoprotective effects of antihypertensive drugs. American Journal of Hypertension 1999;:19S-32S. doi:10.1016/s0895-7061(98)00210-6
- 36Keller E, Hoppe-Seyler G, Schollmeyer P. Disposition and diuretic effect of furosemide in the nephrotic syndrome. Clin Pharmacol Ther 1982;:442–9. doi:10.1038/clpt.1982.187
- 37Parker K, Vincent M, Mohammed M, et al. Diuretic therapy explained. The Pharmaceutical Journal. 2015.https://www.pharmaceutical-journal.com/cpd-and-learning/cpd-article/diuretic-therapy-explained/20067545.cpdarticle (accessed Feb 2021).
- 38Mees EJD. Does it make sense to administer albumin to the patient with nephrotic oedema? Nephrology Dialysis Transplantation 1996;:1224–6. doi:10.1093/ndt/11.7.1224
- 39Kong X, Yuan H, Fan J, et al. Lipid-lowering agents for nephrotic syndrome. Cochrane Database of Systematic Reviews Published Online First: 10 December 2013. doi:10.1002/14651858.cd005425.pub2
- 40Benvenga S, Vita R, Di Bari F, et al. Do Not Forget Nephrotic Syndrome as a Cause of Increased Requirement of Levothyroxine Replacement Therapy. Eur Thyroid J 2015;:138–42. doi:10.1159/000381310
- 41Nephrotic syndrome in adults. National Institute of Diabetes and Digestive and Kidney Diseases. 2014.https://www.niddk.nih.gov/health-information/kidney-disease/nephrotic-syndrome-adults (accessed Feb 2021).