
Shutterstock.com / JL
This content was published in 2009. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
In addition to preventing malnourishment and maintaining normal body function and daily activities, good nutrition is essential in hospital to resist infections and prevent delayed healing of wounds after surgery. As discussed in a previous CPD article(PJ, 15 November 2008, pp571–4), intravenous fluids containing water, glucose and the major electrolytes potassium and sodium can be used to maintain hydration, metabolic activity and organ function when the oral route is either not available or cannot supply adequate quantities of these substances to the body. However, such fluids administered through peripheral cannulae cannot provide sufficient calories. For example, two litres of isotonic (5 per cent) glucose will only provide 100g of glucose or 400kcal — along way short of the normal 1,500–2,000kcal typically required by an adult each day.
Increasing the volumes of fluid delivered to the patient to give adequate calories would lead to fluid overload and subsequent hyponatraemia. In theory, the concentration of glucose could be raised to in-crease calorie load, but infusions of hypertonic solutions of glucose above 10 per cent by the peripheral route will damage the blood vessels used.Even if sufficient calories could be infused through a peripheral vein, fluid regimens do not provide the other essential requirements.
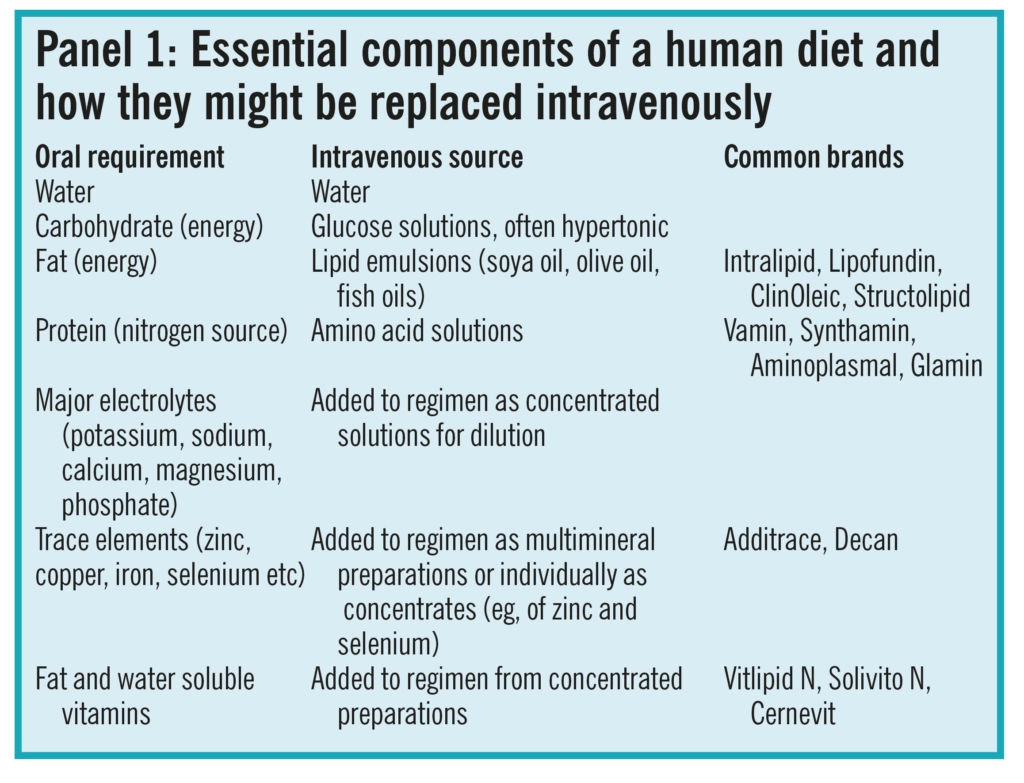
A healthy diet requires a good balance of carbo-hydrates, protein, fat, vitamins, electrolytes and trace elements. These would require intravenous replacement in a patient unable to meet his or her nutritional requirements by the enteral route, whether orally or through feeding tubes. Panel 1 shows how the essential components of the normal diet can be replaced intravenously by mimicking the end products of normal digestion before they are ab-sorbed from the gastrointestinal tract. The term “total parenteral nutrition” refers to the provision of nutrition exclusively by the parenteral route.

Indications for parenteral nutrition
Options other than parenteral nutrition exist for patients who are unable to obtain adequate nutrition by mouth but who have a functioning gut.These include giving sip and enteral feeds through nasogastric, nasojejunal, gastrostomy, or jejunostomy tubes.These options are not only cheaper and carry less risks of patient harm or medical error than par-enteral nutrition but they also maintain healthy gut function until normal feeding is resumed.These options should, therefore, be considered before a deci-sion to initiate parenteral nutrition is taken.
The lack of a functioning gastrointestinal tract for more than about a week has traditionally been the main indication for switching from clear intra-venous fluid regimens to parenteral nutrition. (In young or previously well nourished patients, clear intravenous fluids would usually be sufficient for this period.) In addition, inability to swallow safely, for example, after head and neck surgery, may also indicate parenteral nutrition until another means of feeding can be established. Finally, some patients, such as those with malabsorption problems (eg, Crohn’s disease or short bowel syndrome), fistulae, gut stasis in intensive care situations, or with hyper-emesis in pregnancy may also be given parenteral nutrition in addition to any (sub-optimal) enteral food intake.
Patients with severe malnutrition and weight loss due to vomiting or cancer cachexia may also bene-fit from parenteral nutrition to improve nutritional status before surgery. However, long-term feeding in advanced cancer is controversial because the decision needs to take into account whether continued feeding is likely to extend or improve quality of life or life expectancy.
The production of intravenous amino acid and lipid preparations and the availability of all-in-one parenteral nutrition regimens has made it possible to feed patients intravenously for prolonged periods.
Decisions and considerations
After considering the condition of the patient, the decision to start parenteral nutrition is often based on how many days the patient has been, or is likely to be, unable to maintain an adequate enteral nutrition intake and whether this period can be man-aged without the need for parenteral nutrition.
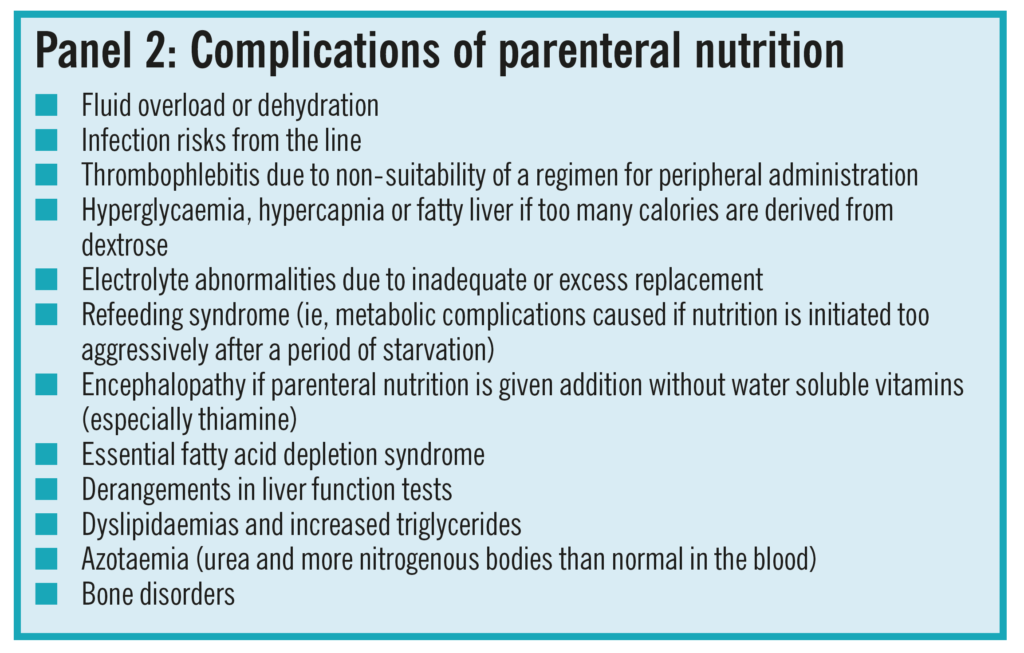
The decision must not be rushed or taken lightly— more harm than good can be done if parenteral nutrition is started without the right expertise.Equally, however, if a patient is likely to require par-enteral nutrition then an assessment and, if appropriate, the initiation of a regimen should take place without delay before malnutrition worsens. A range of complications that can occur from parenteral nutrition, especially if the patient is badly managed, are shown in Panel 2.
The decision to initiate parenteral nutrition is usually collective. Most hospitals have a dedicated nutrition team, consisting of a doctor, a specialist nurse, a pharmacist and a dietitian, that will receive referrals and assess patients for whom any parenteral nutritional support has been requested. The team should determine the most suitable means of feed-ing for the patient. If parenteral nutrition is appropriate, the following issues require consideration when devising the nutrition regimen:
- Fluid volume of regimen
- Energy requirements
- Nitrogen requirement
- Proportion of fat, protein and carbohydrate
- Type of intravenous access available (eg, peripheral, central)
- Whether or not a standard regimen is suitable asa basis for therapy
- Electrolyte and trace element requirements
Fluid volume
Consideration needs to be given to the fluid volume that the patient requires and can tolerate. In the absence of any other fluid input, be-tween 2 and 3L would be reasonable for most patients. However, it is not as simple as that — patients may be receiving other fluids, such as blood, or other infusion fluids (eg, to administer an intra-venous antibiotic, such as clarithromycin or fusidic acid, that requires administration in large volumes of fluid) and these volumes need to be taken into account when determining the fluid volume to be included in the parenteral nutrition regimen.
Furthermore, some patients may require fluid restriction due to other conditions, such as heart or renal failure. On the other hand, other patients may have substantially increased fluid requirements due to excessive or unusual losses from burns, vomiting and diarrhoea, stomas and wound drains.
Energy requirements
A patient’s total energy requirements are calculated using parameters such as sex, age, weight, height and clinical condition. The National Institute for Health and Clinical Excellence recommends that adults should receive between 25 and 35 total kcal/kg/day from par-enteral nutrition.1It is usually preferable to under-feed initially rather than overfeed patients, especially if they are malnourished or in physiological stress.Metabolic complications can arise if a patient is overfed, especially if acutely ill or in the initial period of parenteral nutrition administration. An experienced dietitian will determine the optimum initial energy supply. High risk patients may have to be started on as little as 10kcal/kg/day increasing slowly, over several days, to meet their full needs.
Dextrose
Dextrose supplies energy and forms part of most regimens. It is available in a range of strengths and when infused through a central (large)vein concentrations up to 50 per cent can be ad-ministered. However, even though sufficient calories could be administered in this manner, providing the entire energy needs of a patient from glucose, the addition of appropriate lipid reduces the incidence of hyperglycaemia, hypercapnia, fatty liver and acidosis.
Lipid
In addition to being an energy source, fat also provides essential fatty acids and acts as a carrier for fat-soluble vitamins. Therefore most parenteral nutrition regimens contain fat as an energy source in the form of a lipid emulsion. Fat is calorie dense.For example, 500ml of a 20 per cent lipid solution will provide about 1,000kcal. The use of lipid re-duces the need to use excessive volumes or strengths of glucose, thereby reducing the risk of complications. In addition, the consequent lower osmolality of the feeding solution may permit peripheral rather than central administration of the regimen.
Lipid emulsions were traditionally long chain triglycerides and these are still widely used. More recently, other emulsions have been developed using a mixture of medium- and long-chain triglycerides, and these are claimed to be less likely to cause derangements of liver function tests. Some newer emulsions contain olive oil and even omega-3 fatty acids, postulated to be immunomodulatory.
Nitrogen
Starvation and critical illness can lead to loss of muscle mass so there is a need to provide protein. The addition of an amino-acid solution buffers the suspension and provides nitrogen for protein synthesis. Under metabolic stress, amino-acids can be used as an energy source by the body.Sufficient calories must, therefore, be provided alongside the amino-acids in a parenteral nutrition regimen to prevent them being used for energy. The factors determining a patient’s nitrogen requirement (about 0.2g nitrogen/kg/day according to NICE)[1] are broadly the same as those for energy requirements, as already discussed.
Solutions containing mixtures of both essential and non-essential amino-acids have been available for many years, although there is debate about the best ratios of each amino-acid in the literature.Some specialist solutions are available (eg, those rich in glutamine), which have been shown to be of benefit to critically ill patients.
Standard regimens
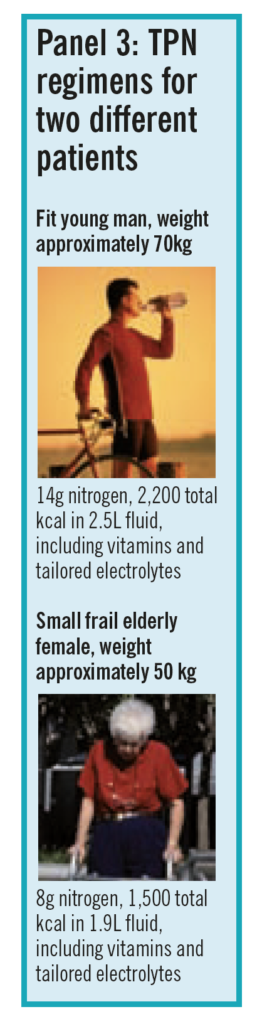
Because the need for energy and nitrogen are determined by similar factors, a small number of standard regimens suffice as the basis of parenteral nutrition for most patients. Standard regimens simplify the prescribing, manufacturing and under-standing of the parenteral nutrition bag by all staff involved. Standard regimens can be adjusted by adding electrolytes, such as sodium, potassium, calcium, magnesium and phosphate in an aseptic environment. Bags usually underestimate electrolyte content to allow for this further individualisation in the light of a particular patient’s needs. Two examples of a typical trust’s most common regimens are shown in Panel 3.
Some patients, however, cannot be managed by parenteral nutrition tailored from such standard regimens. For example, patients receiving sedation with propofol (a lipid emulsion) or with high baseline triglyceride levels, may require fat reduction. Similarly, both fluids and electrolytes may require major reduction in severe renal impairment.
The process of preparing bespoke regimens requires specialist management by the nutritionalsupport team, and assessment by pharmacists of whether the final parenteral nutrition solution will be physically and chemically stable.The lipid emulsion in the regimen becomes unstable:
- When glucose concentrations are either too high or low
- If pH is too low
- If the electrolyte load of the bag is high (especially in regard to divalent cation concentrations)
Prescribing and preparation
Once a decision on initial nutritional and fluid requirements, and whether a standard or bespoke regimen is to be used, has been reached, all the ingredients (as shown in Panel 1) are compounded into a single all-in-one bag that supplies all the nutrients simultaneously and requires only one change each day (under strict aseptic technique by the nursing staff). In the early days of parenteral nutrition lipid, dextrose and amino acid solutions were administered as separate infusions, but this practice is now obsolete because it renders the patient more vulnerable to infections and mistakes. It also made feeding inefficient unless the different ingredient solutions were infused concurrently.
Following incidents in the 1980s, which led to patient deaths, the standards for NHS aseptic unit design, operation, staff training and quality assurance have been considerably tightened and apply to all units, whether preparing parenteral nutrition in batches under a manufacturing licence or dispensing for individual patients. Parenteral nutrition infusion bags are prepared in controlled conditions in a hospital pharmacy or commercial aseptic unit, by specialist staff. The formulation worksheets are pre-pared by different pharmacists to those who check and release these products for use.
Because much parenteral nutrition is prepared to standard regimens, commercial manufacturers pro-vide a range of “multi-chamber” semi-prepared bags to ease preparation. These typically contain the glucose, amino-acids, and lipid in separate compartments that can be mixed by applying pressure(usually by squeezing or rolling gently to burst one compartment into another). When unmixed, these commercially made bags have long shelf-lives at room temperature, and increase efficiency by reducing workload in pharmacy aseptic units. Additional electrolytes and vitamins are then added in the aseptic unit.These shorten the shelf life of a bag to about seven days in a refrigerator and 24 to 48 hours at room temperature during administration.
Patients who require significant adjustment from a standard regimen, or with electrolyte loads lower than those already in the multi-chamber bags, would require the preparation of a product from first principles.
One concern about commercial multi-chamber bags is that they might be used by wards without adequate assessment of the patient by specialists, or without the addition of the vitamins and extra electrolytes required[2]. Some hospitals, hospices and care homes without pharmacy aseptic services might be tempted to add these at ward level but recent guidelines from the National Patient Safety Agency state that non-aseptic additions to any infusion fluid should be avoided wherever possible[3]. Some units use separate IV vitamin infusions if these cannot be added in an aseptic unit.
Administration
Parenteral nutrition is usually initially infused over 24 hours, providing all the nutrients together and allowing efficient use by the body. Longer term or stable patients, especially those requiring only partial nutrition, can often be fed over shorter periods, often overnight, allowing normal activities through the day. Many higher osmolality parenteral nutrition bags are designed for administration through central lines inserted into a major vein, such as the internal jugular or subclavian, where the solution will be rapidly diluted. Central lines for long-term use are often tunnelled under the skin and this reduces infection risk. NICE recommends the use of tunnelled subclavian lines where parenteral nutrition is expected to be required for more than 30 days. An alternative to tunnelled lines for some patients is the use of peripherally inserted central cannulas (PICC)for short- to medium-term use. As the name suggests, these are lines inserted at peripheral sites, usually above the elbow joint then fed up the arm into a larger vein. The advantage of PICC lines is that they can be inserted on the ward by trained nurses, instead of requiring theatre time, a trained doctor and an anaesthetist. PICC lines are also much easier and safer to remove and replace.
Some regimens have a sufficiently low osmolality to enable peripheral administration. A glyceryl trinitrate patch may be applied to the infusion siteand left on for 24 hours — vasodilation reduces the risk of thrombophlebitis in the vein used for administration.
Complications and monitoring
The most common short-term problems from par-enteral nutrition are fluid overload, electrolyte derangements, refeeding syndrome, hyperglycaemia, hyperlipidaemia and infections. Without adequate monitoring, parenteral nutrition can be dangerous and protocols for good practice need to be followed for patient safety. Longer-term problems include impairment of liver function and osteoporosis.Monitoring is especially needed in new and severely unwell patients.
Many parenteral nutrition patients are unwell and vulnerable to infections for reasons in addition to malnutrition. Good general observations of the patient by nurses can identify early signs of an infection (which in intravenous lines could rapidly progress to septicaemia). Initially, temperature, pulse, blood pressure and respiration should be checked four-hourly. The presence of an intravenous line and infusion of a glucose-rich mixture increases the risk of sepsis developing.
If a patient develops a fever on parenteral nutrition, any antipyretics should be withheld and the temperature rechecked within a couple of hours. If the patient still has a fever, feeding is usually stopped and replaced with clear fluids until it is proven that the line is not the source of infection — blood samples taken from the line and peripherally are sent for culture and sensitivity of any bacteria grown. Continuing to feed through an infected line would be dangerous. If a site of infection unrelated to par-enteral nutrition is suspected, appropriate antibiotics may be prescribed empirically. If line infection is suspected, however, the line is generally removed and the tip sent to a microbiology laboratory for culture.
Trusts will have protocols for suspected line infections and since staphylococci are the usual causative organisms most units would initiate teicoplanin or vancomycin therapy, which will also cover methicillin-resistant Staphylococcus aureus (MRSA), although gentamicin will be needed to treat any gram-negative organisms which become more common commensals in intensive therapy units and high-dependency patients. Parenteral nutrition may be restarted if the line culture is negative.
Weight gain during parenteral nutrition should be slow and steady. The patient should be weighed regularly because sudden weight changes may indicate fluid overload or dehydration. Fluid balance charts recording all inputs and outputs from the patient are also invaluable in this regard.
Blood chemistry
Major electrolytes should be checked before starting parenteral nutrition and then initially daily so that the nutrition team can ad-just the electrolyte content of the regimen before compounding. As happens with clear fluids, sodium derangements may reflect fluid overload or dehydration rather than true sodium level in the body.Similarly, hyperkalaemia may be secondary to hyperglycaemia and shifts of potassium from within cells due to a relative deficiency of insulin.Hyperglycaemia is common due to the large amounts of glucose being administered in often physiologically stressed patients and may require the administration of insulin to avoid complications.Hyperglycaemia can be reduced by avoiding over-feeding. Indeed, balanced underfeeding may be better for the patient. Parenteral nutrition should not be stopped abruptly because the rapid removal of the glucose supply while endogenous insulin is being stimulated may lead to rebound hypoglycaemia.
Other tests
Full blood counts, liver function tests, clotting screens and tests for minor electrolytes and trace elements will also be required.These are measured before starting parenteral nutrition and then at least at weekly intervals. Magnesium and calcium levels should initially be checked daily but once the patient is stabilised this can be reduced to twice a week. Patients stable in their own homes often only require monitoring every couple of months.
Summary
The involvement of pharmacists, pharmacy technicians and assistants as part of the multidisciplinary nutrition team, and in aseptic production facilities, is essential to parenteral nutrition being provided safely, efficiently and professionally in all healthcare settings. A future CPD article will discuss the provision of nutrition via nasogastric, nasojejunal, gastrostomy and jejunostomy tubes.
- 1Nutritional support in adults. NICE Clinical Guideline No. 32. NICE. 2006.xxx
- 2Promoting safer use of injectable medicines NPSA patient Safety Alert No. 20. xxx. 2007.xxx
- 3Position statement on use of multi-chamber parentera lnutrition bags for use in adult patients. BritishPharmaceutical NutritionGrou. 2007.xxx
You might also be interested in…
Can pharmacists and dietitians safely prescribe and administer parenteral nutrition?
