This content was published in 2012. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Key points
- Oral antiplatelets include aspirin, clopidogrel, prasugrel, dipyridamole and ticagrelor. Uses include secondary prevention in myocardial infarction, angina and ischaemic stroke (not related to atrial fibrillation).
- Anticoagulants include warfarin, phenindione, acenocoumarol, dabigatran, rivaroxaban and apixaban. Uses include treating or preventing venous thromboembolism and stroke prevention in atrial fibrillation.
- Antiplatelets and anticoagulants can be used together in complex cases. The newer anticoagulants are being investigated for MI and unstable angina.
Despite significant improvements over the past decade in cardiovascular-related mortality, the prevalence of cardiovascular disease has increased, partly due to an ageing population and increasing rates of obesity. As a result, cardiovascular disease continues to be the biggest killer in the UK. In 2010, just under 160,000 deaths in England and Wales were attributed to circulatory disease, which includes athero- and venous thrombotic conditions.
Recent advances in antiplatelet and anticoagulant therapy have resulted in an increasing number of antithrombotic agents for the management of these conditions, providing more choice for clinicians and patients but potentially making treatment decisions more complex.
Thrombosis
Haemostasis is triggered by vascular injury and involves the transition of blood from liquid to solid state. The process comprises three key stages at the site of vascular injury:
- Vasoconstriction — a damaged blood vessel constricts to reduce blood flow
- Platelet plug formation — platelets adhere to the site
- Blood clotting (coagulation through activation of clotting factors)
The coagulation cascade mechanism produces fibrin, which forms a stabilised fibrous polymeric mesh. This traps red blood cells at the site of injury and reinforces the platelet plug. The result is a hard clot (thrombus) that seals off the damaged vessel, reducing blood loss.
Pathologically, the process of haemostasis can be triggered by fissures or ruptures to atherosclerotic plaques within coronary or peripheral vessels. In addition, haemostasis may be initiated in response to extended periods of immobility, prothrombotic states (eg, pregnancy, malignancy) or following surgery, particularly where prosthesis is involved (eg, hip, knee and heart valve replacements). When the haemostatic process is uncontrolled the result is often thrombus formation. The thrombus may cause vascular occlusion at its site of origin, or part or all of the thrombus may be dislodged and occlude another site (embolism) leading to either transient or permanent ischaemia or necrosis.
Differences in blood flow and pressure between the venous and arterial systems result in variation in the composition of thrombi that are formed at different locations. Arterial thrombi tend to be rich in platelet content whereas venous thrombi tend to be fibrin-rich. As such, the primary antithrombotic strategy for arterial thrombosis is antiplatelet therapy and for venous thrombosis the primary strategy is anticoagulation. These strategies may be combined in complex cases (see later).
Reflect
- Why are anticoagulants rather than antiplatelets used for atrial fibrillation?
- What is the optimal duration of dual antiplatelet therapy?
- How do the new generation anticoagulants work? Before reading on, think about how this article may help you to do your job better.
Oral antiplatelets
Antiplatelets work by disrupting key steps in platelet activation via a number of mechanisms, including inhibition of platelet agonists and platelet adhesion or aggregation. Aspirin exerts its antiplatelet activity by irreversibly inhibiting cyclooxygenase 1 (COX-1). In this way it blocks the formation of the precursors of the platelet agonist thromboxane. Although aspirin has a short half-life (of about 20 minutes), because inhibition is irreversible, the effect lasts for the lifespan of the platelet (usually eight to 10 days).
Dipyridamole is thought to work by increasing concentrations of the platelet aggregation inhibitor, cyclic adenosine monophosphate (cAMP). This is achieved by blocking the uptake of adenosine and inhibiting the enzymatic degradation of cAMP. Modified release dipyridamole has a halflife of about 10 hours so is taken twice a day.
The active metabolites of the thienopyridine prodrugs clopidogrel and prasugrel (Efient ▼), reduce platelet activation by non-competitively and irreversibly blocking the binding of adenosine diphosphate (ADP) to P2Y12 receptors on the platelet membrane. In this way ADP induced platelet aggregation is inhibited. Clopidogrel, is metabolically transformed by cytochrome P450 enzymes in the liver into its short-lived active platelet-inhibiting metabolite. Variable metabolic activity of cytochrome P450 enzymes contributes to the observed interindividual variability in the drug’s platelet inhibitory effect. Prasugrel is a more potent inhibitor of ADP than clopidogrel, and is less affected by variation in the expression of different P450 enzymes.
Ticagrelor (Brilique ▼) is a direct reversible P2Y12 receptor antagonist, which, unlike clopidogrel and prasugrel, does not require metabolic activation in the liver. The plasma half-life of ticagrelor is six to 13 hours so twice daily dosing is required.
Oral anticoagulants
Anticoagulants work by directly or indirectly reducing the production of clotting factors and in so doing, limiting the production of fibrin and clot formation. Vitamin K is required for the synthesis of functional clotting factors 2, 7, 9 and 10. During the formation of these factors the reduced form of vitamin K is converted to vitamin K epoxide. This is then recycled back to the reduced form for further synthesis of functional clotting factors in the liver.
Vitamin K antagonist anticoagulants, such as warfarin, phenindione and acenocoumarol, work indirectly by antagonising vitamin K epoxide reductase, the enzyme involved in the recycling. By lowering levels of the reduced form of vitamin K the synthesis of functional clotting factors is inhibited.
After many years of vitamin K antagonism being the only mechanism of action of oral anticoagulants, a range of new oral anticoagulants are now available. The new generation of oral anticoagulants directly inhibit one of two key clotting factors: thrombin and factor Xa. Thrombin is the clotting factor (along with Factor XIII) involved in converting soluble fibrinogen to insoluble polymeric fibrin and is inhibited by dabigatran (Pradaxa ▼). Factor Xa works earlier in the coagulation cascade promoting and activating the generation of thrombin. It is inhibited by rivaroxaban (Xarelto ▼) and apixaban (Eliquis ▼).
How antiplatelets are used
Panel 1 outlines key indications and associated regimens for oral antiplatelets. Primary prevention of cardiovascular events with antiplatelet therapy is no longer routinely recommended.1 Recent evidence indicates the benefit in reduction of non-fatal myocardial infarctions does not outweigh the risk of bleeding and that there is no significant reduction in vascular mortality.2 However, single or dual antiplatelet therapy are recommended for secondary prevention.

A meta-analysis of the Anti-Thrombotic Trialists Collaboration showed that for secondary prevention, the absolute benefits of long-term aspirin monotherapy outweighed the absolute risks of major bleeding in chronic stable angina, unstable angina, MI, stroke and transient ischaemic attack. Furthermore, trials comparing clopidogrel plus aspirin dual antiplatelet therapy with aspirin monotherapy demonstrated the benefit of this combination,3,4 with consistent evidence of a favourable risk-benefit profile in acute coronary syndrome and percutaneous coronary intervention.
Despite aspirin and clopidogrel dual therapy, a significant proportion of patients continue to experience recurrent atherothrombotic events. This observation has led to the exploration of more potent antiplatelets. More recently dual antiplatelet therapy with ticagrelor or prasugrel in combination with aspirin has demonstrated benefit over the clopidogrel-aspirin combination in the management of acute coronary syndrome although this has generally been at the expense of increased bleeding. Prasugrel data have demonstrated a reduction in non-fatal MI rate and stent thrombosis5 and ticagrelor has demonstrated benefit in MI and vascular and total mortality compared with clopidogrel in patients with acute coronary syndrome.6
Treatment duration
The preferred treatment strategy for ST-elevation MI (STEMI) and high risk non-ST-elevation MI (NSTEMI) is revascularisation to restore sufficient blood flow to the affected vessel (reperfusion). The methods used are percutaneous coronary intervention (usually with stents) or coronary artery bypass graft. Revascularisation procedures are coupled with antiplatelet therapy to support plaque stabilisation and inhibit clot formation. The use of drug eluting stents has reduced the risk of arterial restenosis compared with bare metal stents but both methods carry a small risk (≤2 per cent) of stent thrombosis. Drug-eluting stents are particularly associated with late (within one year of insertion) or very late stent thrombosis. This rare but serious complication often presents as large non-fatal MI or death.
There is variation in guideline recommendations on the duration of dual antiplatelet therapy for acute coronary syndrome and for elective percutaneous coronary intervention. National Institute for Health and Clinical Excellence guidelines on secondary prevention of MI advocates a minimum of 28 days therapy but in current practice longer durations are seen. The latest European Society of Cardiology guidance on antiplatelet agents recommends that all acute coronary syndrome patients be treated with 12 months of dual antiplatelet therapy.7 Continuation beyond 12 months should take into account risk factors for further events on an individual basis.
The appropriate duration of dual therapy with aspirin and clopidogrel after elective drugeluting stent insertion has not been confirmed. Current recommendations range between three and 12 months, depending on stent type.7 A number of centres advocate extending dual antiplatelet therapy beyond 12 months to reduce the occurrence of very late stent thrombosis.
On the other hand, in a recent randomised all-comer trial of dual antiplatelet therapy, 2,000 patients requiring stents were randomised to receive aspirin plus clopidogrel for either six months or 24 months.8 The data showed that extending clopidogrel therapy beyond six months did not result in clinical benefit (no difference in all-cause mortality, non-fatal MI, or stroke) but did result in a significant increase in major bleeding. However they must be interpreted cautiously because the study was open-label and of relatively small size.
Because variation is observed in the duration of dual antiplatelet therapy following percutaneous coronary intervention, there needs to be a shift from simple stop dates for therapy to review dates, allowing an individualised assessment and balancing of patients’ ongoing risk for thrombotic events against the increased bleeding risk associated with prolonged therapy.
How anticoagulants are used
Warfarin has been used for a wide range of venous and arterial thrombotic conditions (see PJ 2011;287:251–4). The new generation of oral anticoagulants are being marketed and investigated for a range of indications. Panel 2 lists their current and potential uses.
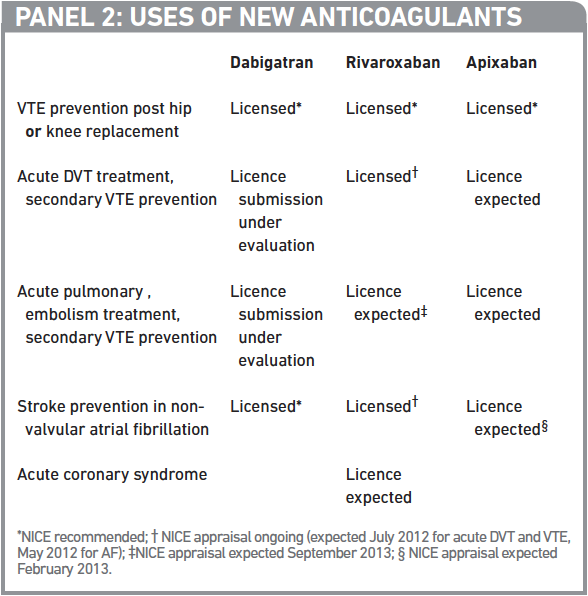
Atrial fibrillation
Atrial fibrillation (AF) results in periods of blood stasis and turbulent blood flow. The result is that fibrin-rich clots formed within the atria can, if dislodged, travel to the brain, leading to a cardioembolic stroke. AF increases the risk of stroke in patients five-fold.
AF-related strokes are associated with a 50 per cent one-year mortality rate and survivors of such strokes are frequently left severely disabled. Only anticoagulant therapy has been shown to reduce AF-related deaths.9 Despite unequivocal evidence of benefit in atrial fibrillation, warfarin continues to be under-prescribed.10
In addition, traditionally, aspirin has been given to patients not regarded as appropriate for warfarin, on the basis that although less effective it is also less harmful. However, evidence suggests that although AF patients do not derive significant benefit from aspirin,10 rates of a major bleed with aspirin may be similar to that of warfarin.11 Similarly, dual antiplatelet therapy does not perform as well as warfarin in terms of clinical outcomes, but has a similar rate of bleeding to warfarin.12 Recently produced guidelines, such as the European AF management guidelines, are calling for the use of aspirin to be significantly reduced or even phased out as an antithrombotic strategy for AF.13
STEVE GSCHMEISSNER/SCIENCE PHOTO LIBRARY
The new oral anticoagulants, dabigatran and rivaroxaban are both licensed within the UK for non-valvular AF. NICE guidance for the use of dabigatran in AF, published last month, recommended dabigatran as an alternative to warfarin within its licensed indication.14 A P&MM article (pp521–2) discusses what this means for patients, prescribers and the NHS.
Acute coronary syndrome
The new oral anticoagulants are also being investigated for acute coronary syndrome (ie, MI, unstable angina), for use with dual antiplatelet therapy. In the ATLAS ACS 2–TIMI 51 trial, when low dose rivaroxaban was added to aspirin and clopidogrel there was a reduction in all-cause and cardiovascular mortality compared with placebo. However the risk of major bleeding and intracranial haemorrhage (but not fatal bleeding) was increased.
Dabigatran and apixaban have also been investigated (in combination with antiplatelet therapy15), but efficacy and safety profiles have been unfavourable so, for now, formal consideration of them for acute coronary syndrome has ceased.
Complex cases
As alluded to above, in some cases, oral antiplatelet and anticoagulation therapy is combined. AF is a manifestation of cardiovascular disease so it is not surprising that it occurs in up to 21 per cent of patients with acute coronary syndrome.16 In addition, 5–10 per cent of patients who have a stent will have a concomitant indication (eg, mechanical heart valve prosthesis, previous thromboembolism) for oral anticoagulation. Decisions will need to be made about the appropriateness of “triple therapy” with warfarin and dual antiplatelet therapy.
Specific recommendations for the management of AF in patients with acute coronary syndrome are predominantly based on consensus because data are lacking. The general rule is that when triple therapy is deemed necessary it should be used for the shortest possible time. In addition, it may be appropriate to maintain the patient’s international normalised ratio at the lower end of the specified target range.
In general, patients with stable coronary artery disease (arbitrarily defined as no event for ≥1 year) taking long-term warfarin should not routinely be prescribed antiplatelet therapy.16 Patients with more recent cardiovascular events should be risk assessed and, as appropriate, considered for triple therapy with warfarin, aspirin and clopidogrel in the short term (three to six months) or longer if bleed risk is low. After that a patient can be maintained on warfarin in combination with one antiplatelet if necessary and appropriate.
For AF patients requiring elective percutaneous coronary intervention, a bare metal stent is preferred because dual antiplatelet therapy can be limited to four weeks compared with around 12 months with a drug-eluting stent.
There is limited experience of triple therapy with the newer oral antiplatelets and oral anticoagulants so it would seem prudent to avoid such combinations until evidence becomes available on specific regimens and associated doses and durations.
Monitoring
Before initiating any oral antithrombotic therapy, the benefits of treatment must be weighed against the risks of bleeding. There are a number of condition specific risk assessment tools for determining the thrombotic and bleed risks that can be used to guide management decisions (eg, whether to start antithrombotic therapy, if so what drugs, duration of therapy and whether gastroprotection is required).
It is good practice before initiating oral antiplatelets or oral anticoagulants to undertake a full coagulation screen (including INR and activated partial thromboplastin time [aPTT]), full blood count, and renal and liver function tests to identify any inherent bleeding issues.
In October 2011 the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) recommended an update to the product information for dabigatran following reports of increased bleeding and bleeding fatalities in the elderly and those with poor renal function.
The recommended updated product information includes advice that renal function should be assessed in all patients before starting treatment (dabigatran is 85 per cent renally metabolised). During treatment, renal function should be assessed at least yearly in patients over 75 years of age and in those with known renal impairment. It should also be assessed whenever a decline in function is suspected.
Rivaroxaban and apixaban are renally metabolised to a lesser extent but caution (eg, dose reduction) and, as appropriate, close monitoring in moderate to severe renal impairment should still be applied.
The newer antiplatelets are more potent than aspirin and clopidogrel but come with increased bleeding rates, while the new generation of oral anticoagulants range from being non-inferior to superior to warfarin and offer the benefit of less intracranial haemorrhage with variable major bleed rates (ranging from equivalent to less bleeding versus warfarin). For side effects, cautions, contraindications and interactions see the latest BNF and summaries of product characteristics.
Platelet function
Routine monitoring of platelet function is not undertaken although there has been some interest in monitoring of platelet function for patients on aspirin and clopidogrel when there is concern of non-responsiveness. With respect to aspirin, current tests lack sensitivity in measuring COX-1 inhibition, so there is currently no role for monitoring the response to therapy other than to check patient adherence regularly.
There is a stronger case for platelet function tests that assess the efficacy of clopidogrel therapy. This is because, as a prodrug, clopidogrel needs to undergo metabolic activation. CYP450 polymorphisms affect the metabolic activation of clopidogrel resulting in variable pharmacodynamic response. At present tests to assess platelet function in response to clopidogrel are not widely available, but are being investigated. In addition, genotyping to assess patient’s ability to metabolise clopidogrel is also being investigated.
Data exploring the use of platelet function testing for prasugrel and ticagrelor is limited. There is debate about the usefulness of these tools.
Coagulation
Due to their unpredictable pharmacokinetics and narrow therapeutic windows, routine coagulation monitoring using INR is essential for vitamin K antagonist anticoagulants. Although the new oral anticoagulants demonstrate predictable and stable pharmacokinetics and do not require routine coagulation monitoring, there are some instances where assessment of coagulation is appropriate (eg, pre-operatively, suspected over-anticoagulation). Currently, commercial monitoring is not widely available for the new agents, making accurate quantitative determination of anticoagulation challenging. The aPTT provides a useful qualitative assessment of anticoagulant activity and may be used to assess overanticoagulation in patients taking dabigatran. In addition, thrombin time (TT) and ecarin (a prothrombin activator) clotting time may also serve as qualitative markers.
In terms of quantitative assessment of dabigatran activity, the manufacturers suggest measurement of dabigatran concentration using a diluted TT assay such as the Hemoclot assay.
With respect to factor Xa inhibitors (ie, rivaroxaban and apixaban), chromogenic antifactor Xa assays may be used to assess plasma concentrations. Evidence is also emerging about the usefulness of standardised tests of prothrombin time using appropriate thromboplastins for the quantitative assessment of anticoagulation with factor Xa inhibitors. In addition, aPTT may be used to identify overanticoagulation qualitatively.
When interpreting assays for the newer oral anticoagulants it is important to know the timing of the blood test relative to the last dose because results vary widely depending on when this dose was taken. This is unlike INR monitoring for warfarin where the timing is not as relevant because the drug has a long halflife and prolonged action on clotting factors.
Practice points
Reading is only one way to undertake CPD and the regulator will expect to see various approaches in a pharmacist’s CPD portfolio.
- Talk to your patients prescribed antiplatelets. Do they know what they are using them for? Is the duration of therapy appropriate?
- Find out more about the practice implications of recent NICE recommendations on the newer anticoagulants by reading the P&MM article on pp521–2.
- Discuss with a colleague: what questions patients are likely to ask about the newer antiplatelets and anticoagulants, and what your answers would be.
Consider making this activity one of your nine CPD entries this year.
References not currently available online.
You might also be interested in…

UK Clinical Pharmacy Association launches pharmacogenomics guidance
Health news round-up: cardiovascular disease, women’s health and the potential for new sepsis treatment
