This content was published in 2011. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Summary
It is crucial that a person suffering a stroke receives rapid diagnosis and management in an acute stroke unit. The treatment of stroke in the acute phase will depend on the type of stroke — broadly, whether the stroke is ischaemic or haemorrhagic.
The management of ischaemic stroke can involve thrombolysis, providing the patient can be treated soon after the onset of symptoms. Managing haemorrhagic stroke focuses on correcting any coagulation abnormalities. All patients should have their fluid status, blood pressure, blood glucose, temperature and oxygen saturation monitored closely.
The most important factor in the treatment of stroke is time — early management is vital for optimising patient outcomes and reducing disability caused by stroke. Following initial assessment, patients who are likely to have suffered from a stroke recently should be referred immediately to a hyperacute stroke unit (see Box 1) for rapid investigation and multidisciplinary management, including early rehabilitation and frequent monitoring.1–3

Specific treatment for stroke depends on the underlying pathology (ischaemic versus haemorrhagic), cause (eg, artery stenosis, cardiac embolism or venous thrombosis) and site of the stroke.
Ischaemic stroke
Intravenous thrombolysis
Intravenous thrombolysis with the recombinant human tissue plasminogen activator alteplase (rt-PA) within 4.5 hours of onset of ischaemic stroke can significantly improve patient outcomes.4 Although other thrombolytic drugs have been investigated for the treatment of ischaemic stroke, alteplase has the largest body of clinical evidence to support its use and, currently, is the only medicine licensed for this indication.
Alteplase has a short half-life of approximately five minutes and is administered as an initial IV bolus (10% of the total dose) followed by the remaining 90%, which is infused over 60 minutes. The dose is calculated using patient weight (0.9mg/kg) up to a maximum of 90mg. This dose is different from that used for thrombolysis in myocardial infarction or pulmonary embolism.
There are strict exclusion criteria for thrombolysis to ensure that it is not given to patients with a high risk of serious bleeding or where the risk of haemorrhage outweighs the benefits of thrombolysis (outlined in the summary of product characteristics for alteplase).
Alteplase should only be administered within a well organised stroke unit that has immediate access to the required facilities (eg, imaging equipment) and staff trained in interpreting images, delivering thrombolytic therapy and monitoring for complications.2 Protocols should be available for the management of thrombolysis.2
The phrase “time is brain” is commonly used to highlight the point that outcomes are improved when treatment is started quickly. Treatment is twice as effective if it is administered within 1.5 hours of the onset of stroke symptoms compared with treatment in the 1.5–3 hour window. The outcomes of treatment are poorer still when alteplase is administered after three hours. Despite this, a modest improvement in clinical outcomes can occur when alteplase is given up to 4.5 hours after symptom onset.4 Thrombolysis in this later time window is associated with more symptomatic intracranial haemorrhage, but can be given based on clinical judgement. The third international stroke trial (IST-3) is currently investigating thrombolysis with alteplase for patients up to six hours after the onset of stroke symptoms.5
The 2010 “National sentinel stroke audit”, which assessed stroke services in England, Wales and Northern Ireland, showed that 5% of stroke patients had received thrombolysis although 14% had been eligible (based on criteria from the National Institute for Health and Clinical Excellence).6 Nevertheless, it also showed that the rate of thrombolysis had increased from 1.8% in 2008, mostly due to investment in hyperacute stroke services that operate 24 hours a day, seven days a week.6
Mechanical clot retrieval
Mechanical clot retrieval by experienced interventional neuroradiologists can be considered for patients unsuitable for thrombolysis.3 Research has shown that mechanical clot retrieval with the MERCI (mechanical embolus removal in cerebral ischaemia) device within eight hours of stroke onset is associated with higher rates of restored blood flow (ie, recanalisation) than historical controls.3
Antiplatelet medicines
All patients with an acute ischaemic stroke, including those with atrial fibrillation, should be prescribed aspirin 300mg daily as soon as possible after the onset of symptoms.2 Ideally, this should be given orally but if the patient is dysphagic administration using an enteral feeding tube or via the rectal route may be more appropriate.2 If a patient has received thrombolysis, antiplatelet therapy should be not be started until 24 hours have passed and repeat imaging has ruled out haemorrhagic transformation of the infarct. Aspirin treatment should be continued for up to 14 days,2 or until a plan for long-term secondary prevention with an antiplatelet medicine or an anticoagulant is decided (see https://pharmaceutical-journal.com/article/ld/stroke-long-term-management).
Statin therapy
Following an acute ischaemic stroke, patients who were already taking a statin should continue to do so.2,3 However, for patients who were not previously taking a statin, one should be started 48 hours after the onset of symptoms — starting a statin immediately is not recommended.2
Haemorrhagic stroke
Haemorrhagic stroke will sometimes require neurosurgical intervention,3 but is usually managed medically.
Correcting coagulopathy
Patients with a coagulation factor deficiency or thrombocytopenia should receive appropriate factor replacement therapy or platelets, respectively.7
Patients taking oral anticoagulants constitute 12–14% of patients who suffer haemorrhagic stroke.7 For patients with oral anticoagulant-associated haemorrhagic stroke and an international normalised ratio >1.4, the oral anticoagulant should be discontinued for at least 10–14 days and their INRs should be normalised using a combination of prothrombin complex concentrate and intravenous vitamin K as soon as possible.2 Coagulation status should be monitored closely and a haematology review obtained in the acute phase. Prothrombin complex concentrates, such as Beriplex or Octaplex, will normalise clotting within minutes, and are generally preferred over fresh frozen plasma because of a quick infusion time and lower risk of allergic reactions.7 Whether or not oral anticoagulant therapy should be restarted (and when this should occur) depends on the perceived risks of thromboembolic occlusion and haemorrhagic stroke recurrence.
Statin therapy
In the acute phase following haemorrhagic stroke, statin therapy is associated with increased risk of haemorrhage, and is not routinely recommended. See accompanying article for information on the use of statins in the long-term management of haemorrhagic stroke.3
Other stroke subtypes
Cervical artery dissection
Dissection of the extracranial carotid and vertebral arteries is a relatively uncommon cause of stroke.3 These patients are at a high risk of early recurrent stroke, mostly within the first month, and this risk is probably highest in the first week.8 Evidence comparing anticoagulation with antiplatelet therapy in cervical artery dissection is lacking.8 Likewise there is insufficient evidence on the safety or efficacy of thrombolysis and stenting in cervical artery dissection. Therefore, patients with stroke secondary to acute arterial dissection should be treated with either anticoagulants or an antiplatelet medicine, ideally in the context of a clinical trial.2
Cerebral venous thrombosis
Cerebral venous thrombosis is rare and accounts for 0.5% of all strokes.9 Unless it is contraindicated, patients with cerebral venous thrombosis, including those with secondary cerebral haemorrhage, should receive full-dose anticoagulation with unfractionated heparin2 or low molecular weight heparin, followed by warfarin therapy (target INR 2–3).2,3,9
Treatment and monitoring for all patients
Swallowing assessment
All patients should have their ability to swallow assessed within 24 hours of stroke onset to ensure that they can safely swallow medicines, food and liquids. Some patients will require alternative routes of drug administration, nutrition and hydration.2
Fluid replacement
Fluid balance should be assessed routinely, and an appropriate intravenous fluid regimen initiated, for all patients who have suffered a stroke. To prevent hyperglycaemia normal saline infusions are preferred over glucose-containing preparations.3 There is no evidence that using plasma expanders (dextran, hydroxyethyl starch or albumin) has any benefit over standard fluid replacement.3
Blood pressure
Patients with high, low or greatly fluctuating blood pressure in the first 24 hours following an ischaemic stroke are more likely to have poorer outcomes.10 This is possibly due to cerebral hypoperfusion (caused by low blood pressure) and cerebral oedema or haematoma expansion (caused by high blood pressure).7 Despite this, evidence regarding optimal blood pressure strategies in the acute phase of stroke, and how these translate into patient outcomes, is lacking.11
Potter and colleagues reported that lisinopril and labetalol use within 36 hours of stoke onset did not increase serious adverse events and reduced three-month mortality by half.12 However, the recent SCAST trial reported that candesartan use in acute stroke patients with hypertension did not improve vascular outcomes during the first six months, and may be associated with poorer functional outcomes.13 In the COSSACS trial, continuation of antihypertensive medication within 48 hours of acute stroke was not associated with improved outcome or increased adverse events.14
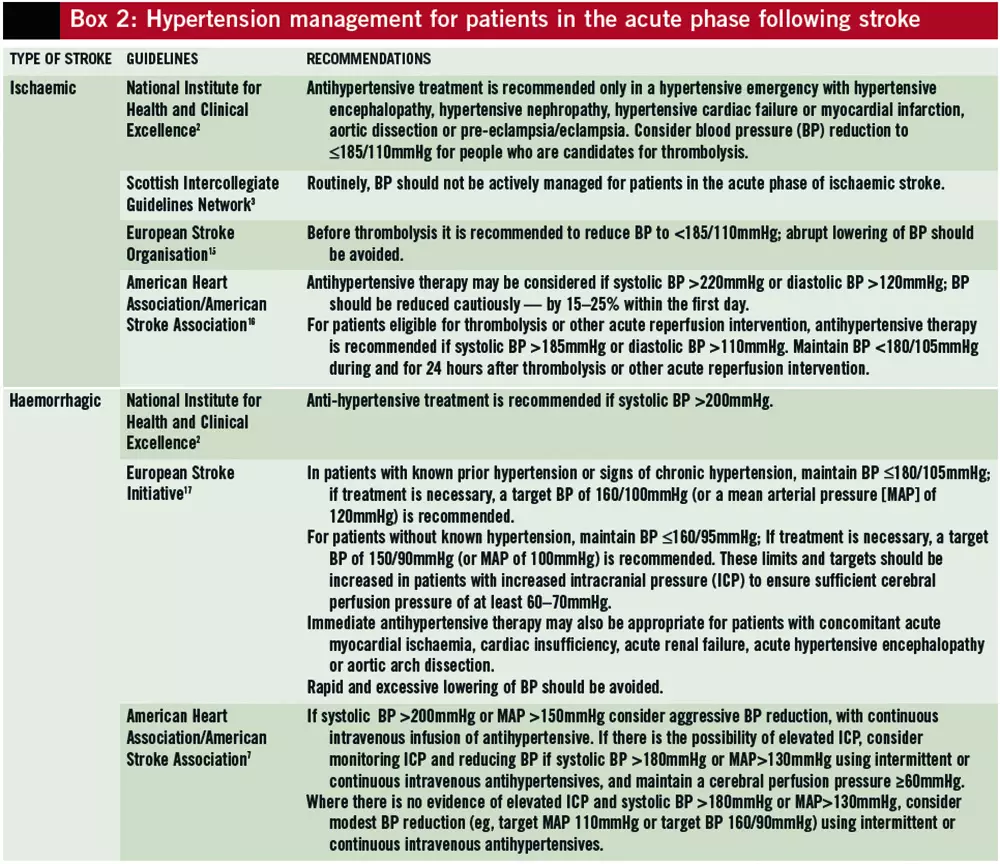
Many patients have spontaneous declines in blood pressure over the first week after stroke,10 and active blood pressure management is not recommended routinely, except in hypertensive emergencies.2,3 Cautious blood pressure reduction of 15–25% in the first 24 hours of stroke onset may be considered if blood pressure exceeds 220/120mmHg in ischaemic stroke, or if systolic blood pressure exceeds 160mmHg for patients with haemorrhagic stroke. Hypertensive patients who are potential candidates for thrombolysis should have their blood pressure lowered to £185/110mmHg before thrombolysis and kept below 180/105mmHg for at least 24 hours after thrombolysis to reduce the risk of symptomatic haemorrhagic transformation.
If treatment is indicated, intravenous antihypertensive drugs with a short half-life should be used first line. In UK hyperacute stroke units, labetalol, glyceryl trinitrate and, occasionally, sodium nitroprusside are used. Sublingual nifedipine should not be used due to the risk of an abrupt decrease in blood pressure.15 Recommendations for the management of hypertension in the acute phase of stroke are summarised in Box 2.
Low blood pressure can usually be raised by adequate rehydration with saline infusions, but patients with low cardiac output occasionally require inotropic support.15
Blood glucose
Hyperglycaemia in the acute phase of stroke is common, even for non-diabetic patients, possibly as a stress response.3,15,16 It is associated with larger infarcts and poorer functional outcomes.3,15,16 Following thrombolysis, hyperglycaemia may also be associated with increased risk of haemorrhagic transformation of ischaemic stroke.15
In the acute phase, patients’ blood glucose should be controlled tightly, aiming for a blood glucose concentration of 4–11mmol/L.2 If required, this can be achieved using continuous infusions of insulin, the rates of which are adjusted based on patients’ blood glucose readings.2 These insulin infusions can be co-administered with an infusion of 5% glucose (given at a constant rate) to avoid hypoglycaemia. Avoiding glucose solutions for fluid replacement in the acute phase of stroke may also help to avoid high blood glucose levels.15
Oxygen
Theoretically, oxygen therapy could potentially reverse hypoxia in acute ischaemic stroke through increased cerebral oxygenation and reduced cerebral oedema. However, high doses of oxygen could also be harmful by increasing oxidative stress through increased oxygen free-radical production.18
In a quasi-randomised study of oxygen therapy in nonhypoxic acute stroke patients, 100% normobaric oxygen at 3L/min for 24 hours via nasal catheter did not improve patient outcomes but increased mortality among patients with minor or moderate stroke.18
Oxygen therapy (2–6L/min via nasal cannulae)19 should only be considered in acute stroke patients if oxygen saturation falls below 95% (or below 92% according to American guidelines).2,15,16 The British Thoracic Society recommends using targeted oxygen therapy in all acutely ill patients.19 The usual target oxygen saturation of 94–98% is appropriate for most patients, but a target of 88–92% should be used for patients at risk of hypercapnic respiratory failure, for example those with chronic obstructive pulmonary disease.19 The ongoing “Stroke oxygen study” is investigating fixed-dose supplemental oxygen in the first 72 hours post-stroke.
There is no good evidence that hyperbaric oxygen improves clinical outcomes for patients with acute stroke; it should not be used outside of clinical trials.3
Temperature
Increased body temperature in the acute phase of stroke is associated with poor outcome and the cause of the elevated temperature should be investigated and treated where appropriate.3,15,16 Although no evidence exists that either physical cooling or the use of antipyretic medicines to lower body temperature affects outcomes, it is common practice to treat raised body temperature (>37.5C) with paracetamol.15,16
Venous thromboembolism prevention
In the acute phase following a stroke, patients are at increased risk of venous thromboembolism (VTE). Early hydration and early mobilisation can reduce the risk of VTE. Antiembolism stockings should not be used for stroke patients because they have been shown to increase adverse events without reducing rates of VTE.2,20
Pharmacological thromboprophylaxis is not recommended routinely for acute stroke patients since the benefit of VTE reduction is offset by an increased risk of bleeding.3 However, some patients have a particularly high risk of VTE and larger trials are needed to study the use of low-dose heparin for such patients. For patients in whom the risk of VTE outweighs the risk of haemorrhagic transformation, prophylactic doses of low molecular weight heparins should be considered in preference to unfractionated heparin, except in renal failure, and reviewed regularly.2,3,15,21
For patients who have a high risk of VTE and who have suffered a haemorrhagic stroke, or who are at high risk of bleeding, the use of foot impulse or intermittent
References
- Department of Health. National Stroke Strategy. December 2007. www.dh.gov.uk (accessed 4 March 2011).
- National Institute for Health and Clinical Excellence. Stroke: diagnosis and initial management of acute stroke and transient ischaemic attack. July 2008. www.nice.org.uk/cg68 (accessed 4 March 2011).
- Scottish Intercollegiate Guidelines Network. Management of patients with stroke or TIA: assessment, investigation, immediate management and secondary prevention. December 2008. www.sign.ac.uk/guidelines/ fulltext/108/index.html (accessed 4 March 2011).
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New England Journal of Medicine 2008;359:1317–29.
- The third international stroke trial (IST-3). www.dcn.ed.ac.uk/ist3 (accessed 24 April 2011).
- Royal College of Physicians. National sentinel stroke audit 2010 — public report. March 2011. www.rcplondon.ac.uk/resources/ national-sentinel-stroke-audit (accessed 24 April 2011).
- Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral haemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010;41:2108–29.
- Menon R, Kerry S, Norris JW, et al. Treatment of cervical artery dissection: a systematic review and meta-analysis. Journal of Neurology, Neurosurgery and Psychiatry 2008;79:1122–7.
- Saposnik G, Barinagarrementeria F, Brown RD, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke (online 3 Feb 2011). http://stroke.ahajournals.org (accessed 14 April 2011).
- Geeganage CM, Bath PM. Relationship between therapeutic changes in blood pressure and outcomes in acute stroke: a metaregression. Hypertension 2009;54:775–81.
- Geeganage C, Bath PMW. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database of Systematic Reviews 2008; issue 4.
- Potter JF, Robinson TG, Ford GA, et al. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebocontrolled, double-blind pilot trial. Lancet Neurology 2009;8:48–56.
- Sandset EC, Bath PMW, Boysen G, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet 2011;377:741–50.
- Robinson TG, Potter JF, Ford GA, et al. Effects of antihypertensive treatment after acute stroke in the Continue Or Stop post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurology 2010;9:767–75.
- The European Stroke Organisation Executive Committee and the ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovascular Diseases 2008;25:457–507.
- Adams HP, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke. Stroke 2007;38:1655–1711.
- The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Recommendations for the management of intracranial haemorrhage — part 1: spontaneous intracerebral haemorrhage. Cerebrovascular diseases 2006;22:294–316.
- Ronning OM, Guldvog B. Should stroke victims routinely receive supplemental oxygen. A quasi-randomised trial. Stroke 1999;30:2033–7.
- O’Driscoll BR, Howard LS, Davison AG. Guideline for emergency oxygen use in adult patients. Thorax 2008;63(Suppl VI):vi1–68.
- The CLOTS Trials Collaboration. Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009;373:1958–65.
- National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. January 2010. www.nice.org.uk/cg92 (accessed 14 March 2011).


