Dr Linda Stannard, UCT / Science Photo Library
Vulvovaginal candidiasis, or thrush, is the most common cause of vaginitis (inflammation of the vagina) and vulvovaginitis (inflammation of the vulva and vagina region). Candidiasis is typically caused by an overgrowth of the fungal yeast Candida albicans. The National Institute for Health and Care Excellence (NICE) states that C. albicans accounts for 80–92% of cases; C. glabrata is responsible for a further 5% of cases; and C. tropicalis, C. parapsilosis, C. krusei, C. kefyr, C. guilliermondii and Saccharomyces cerevisiae account for most of the remaining cases[1].
Candidiasis is very common, with 70–75% of women reportedly experiencing the condition at least once and 40–50% of women reportedly experiencing it two or more times throughout their lives[2],[3]. These figures, although widely claimed in scientific literature, are estimates based on expert opinion from the 1970s[4], with accurate incidence and prevalence rates currently lacking in the UK. However, it is estimated that Candida species could be isolated from 20% of vaginal swabs from otherwise healthy, asymptomatic women of reproductive age at any one time[5].
Although thrush can affect other areas of the skin, such as armpits, groin and mouth (oral thrush), this article will focus mainly on the diagnosis and treatment of genital thrush.
Risk factors
The vaginal microbiome is diverse, however, most women have lactic acid-producing Lactobacillus species, which accounts for the acidic pH range of the vagina[6]. The composition of microflora is also dynamic, with increased change during menstruation and sexual activity[7]. As the majority of women are thought to be colonised with Candida species without obvious symptoms at some point in their lives, it has been proposed that the dynamic nature of the vaginal microbiome is a critical factor that leads to colonisation by Candida, as well as contributing to commensal
Candida species becoming pathogenic[5]. The following risk factors have been identified as leading to colonisation and/or symptomatic candidiasis:
- Genetics (e.g. black British, familial history);
- Changes in circulating hormones (e.g. pregnancy, combined oral contraceptive pill/hormone replacement therapy, menstruation);
- Immunosuppression (e.g. HIV, corticosteroids);
- Diabetes (i.e. poorly controlled [see Box 1]);
- Vulvar dermatosis;
- Antibiotics;
- Sexual activity (e.g. intercourse, orogenital contact);
- Changes in vaginal pH[1],[5].
Box 1: Thrush and diabetes
Thrush is more common in people with diabetes because high blood sugar levels lead to better conditions for the yeast to grow. Pharmacists and healthcare professionals should be aware of this and inform people with diabetes that they are more at risk of vaginal, penile or oral thrush. They should offer patients self-care advice, if appropriate, during medicine use reviews.
Sources: www.nhs.uk/common-health-questions/pregnancy/can-thrush-harm-my-baby-during-pregnancy/ and cks.nice.org.uk/candida-female-genital#!scenario:4
Symptoms and differential diagnosis
The most common causes of vaginitis in symptomatic women are bacterial vaginosis (BV), vaginal candidiasis (most commonly a C. albicans strain) and trichomoniasis (a protozoal infection). Thrush symptoms and signs are very common, but the sensitivity and specificity of any single sign or symptom are suboptimal for diagnosis. The following self-reported symptoms are typical[5],[8]:
- Pruritus (sensitivity 50–91%; specificity 47–64%);
- Cheesy discharge (sensitivity 65%; specificity 73%);
- Irritation (i.e. redness or swelling) (sensitivity 20–28%; specificity 86–92%);
- Another yeast infection (sensitivity 35%; specificity 95%).
Physician-reported ‘curdy’ discharge has a relatively high predictive value of 72% sensitivity and 100% specificity.
pH testing could be used to help assess the cause of symptoms; candidiasis (pH≤4.5), BV (pH>4.5) or Trichomonas vaginalis (pH>4.5)[1].
Thrush is typically non-odorous; self-reported malodour that is ‘fishy’ has a sensitivity of 46% and specificity of 45% for BV. Physician-reported odour of ‘high cheese’ reaches a sensitivity of 78% and specificity of 75% for BV. Other symptoms and signs of BV include physician-reported yellow discharge with sensitivity of 30–89% and specificity of 85–93%[8].
Classification of thrush
To help support treatment choice, vulvovaginal candidiasis has been classified in NICE guidance as uncomplicated or complicated[1].
Uncomplicated vulvovaginal candidiasis is defined as:
- Sporadic or infrequent;
- Mild to moderate;
- Likely to be owing to C. albicans;
- Is not associated with risk factors (e.g. pregnancy or poorly controlled diabetes).
Whereas, complicated vulvovaginal candidiasis includes:
- Recurrent infection — defined as four or more documented episodes in one year, with at least partial resolution of symptoms between episodes;
- Severe infection;
- Infection with yeasts other than C. albicans;
- Infection during pregnancy;
- Infection in women with uncontrolled diabetes, women with immunocompromising conditions (e.g. HIV infection) and women taking immunosuppressive drugs (e.g. systemic corticosteroids).
Microscopy is generally not required for uncomplicated thrush; however, complicated cases, such as recurrent infection, may require laboratory testing to confirm the diagnosis[1].
Treatment
Azole therapies are fungistatic agents and are considered to be the crux of therapy[9],[10]. They act by inhibiting Candida yeast cells from transforming into hyphae[9]. Various azoles are available, including clotrimazole, econazole, fenticonazole, fluconazole, itraconazole and miconazole[9],[10]. Many of these pharmacological agents are available in different formulations and do not require a prescription (see Table 1)[11],[12].
| Drug | Formulation and legal classification | Dosage regimen |
|---|---|---|
| P: pharmacy medicine; POM: prescription-only medicine. Information presented as per National Institute for Health and Care Excellence and British Association for Sexual Health and HIV guidelines[1],[10]. | ||
| Clotrimazole | Pessary [P] | 200mg for three nights; course can be repeated once, if necessary OR 100mg for six nights; course can be repeated once, if necessary OR 500mg for one night; dose can be repeated once, if necessary |
| Cream (10%) [P, POM] | 5g for one dose, one applicatorful to be inserted into the vagina at night; dose can be repeated once, if necessary | |
| Econazole | Pessary [POM] | One pessary for one dose, pessary to be inserted at night; dose can be repeated once, if necessary OR One pessary daily for three days, pessary to be inserted at night; dose can be repeated once, if necessary |
| Cream [P, POM] | One applicatorful daily for at least 14 days, dose to be inserted vaginally at night; and applied daily (to the skin) for at least 14 days, to be applied to vulva at night; course can be repeated once, if necessary | |
| Fenticonazole | Capsule [POM] | 200mg daily for three days OR 600mg daily for one dose, to be inserted at night |
| Cream [POM] | One applicatorful twice daily for three days | |
| Fluconazole | Capsule (oral) [P, POM] | 150mg for one dose |
| Itraconazole | Capsule (oral) [POM] | 200mg twice daily for one day |
| Miconazole | Capsule [POM], discontinued | One capsule daily, ovule to be inserted at night as a single dose; can be repeated once, if necessary |
| Cream [POM] | One applicatorful daily for 10–14 days OR One applicatorful twice daily for 7 days; course can be repeated once, if necessary | |
| Nystatin (combination with dimeticone) — used for external candidiasis | Cream [POM] | To be used three times daily until symptoms clear |
Short courses (1–3 days) of topical azoles are usually effective for the treatment of uncomplicated thrush and are more beneficial than nystatin[3]. Nystatin, a polyene macrolide antibiotic, may also be used in the treatment of Candida infections, including thrush[9],[10]. For uncomplicated thrush, topical and oral azoles are equally effective[10],[13],[14]; hence, choice of formulation should be based on the patient’s preference and the availability of the product[10].
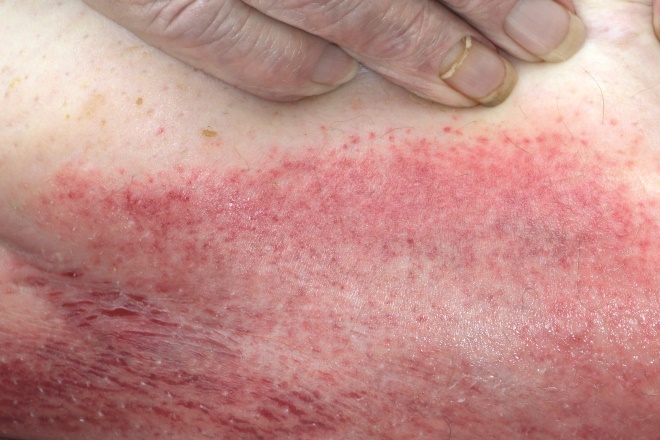
Figure 1: Rash and fungal infection in a patient with diabetes
Source: Science Photo Library
Patients with diabetes are more likely to get thrush, especially if their diabetes is poorly controlled.
For more clinical images on thrush, see NHS Choices.
Patient characteristics
The age of the patient should be considered[1]. The use of topical agents may be less acceptable and more difficult to administer for women aged over 60 years. On the other hand, topical agents are the treatment of choice for girls aged 12–15 years, as oral and intravaginal formulations are not recommended. Pharmacists may prefer to refer girls in this age group to a medical practitioner.
A further factor that may influence treatment choice is co-administration with a patient’s regular medicines. Azoles and nystatin can interact with other medicines. Even topical azoles can lead to severe interactions with prescribed oral therapy. For example, topical miconazole, a potent cytochrome P450 enzyme inhibitor, interacts with warfarin therapy and can lead to large changes in international normalised ratio (INR). Hence, co-administration is not recommended, except in cases where close monitoring of INR is possible[15],[16].
When discussing treatment options with patients, pharmacists and healthcare professionals need to inform them that topical thrush treatment can damage latex condoms and diaphragms; therefore, alternative contraception or treatment formulations may be more appropriate[10]. However, oral formulations are not recommended for pregnant women (see Box 2), for whom only topical azoles should be used[3],[17].
Box 2: Thrush and pregnancy
Pregnancy can increase the chances of women developing thrush; however, it is not harmful to the baby and can be treated with topical treatments and pessaries. Pharmacists and healthcare professionals should advise pregnant women to return if their symptoms have not resolved within 7–14 days.
Women of childbearing age should be advised that thrush will not affect their chances of getting pregnant.
Sources: NHS Choices and National Institute for Health and Care Excellence clinical knowledge summaries on Candida — female genital
Side effects
Therapy with azoles is generally well tolerated; however, topical azoles may cause local side effects (e.g. irritation at the site of application), while oral azoles may cause gastrointestinal symptoms (e.g. nausea) and headaches
[3],[9].
Recurrent or persistent thrush
Typically, recurrent thrush refers to four or more mycologically proven episodes in a 12-month period; however, there is a lack of consensus on the exact number of episodes required for a diagnosis of recurrent thrush, with some definitions requiring only three episodes per year[3],[18]. Women who experience recurrent or persistent thrush that is still present after treatment require evaluation and testing to rule out differential diagnoses[3]. For women with recurrent thrush, a longer duration of therapy may be recommended at the initial presentation. For example, the initial treatment may include up to 14 days of topical therapy or three doses of oral therapy over 1 week[3]. After initial therapy, the first-line maintenance regimen involves weekly 150mg oral fluconazole for a 6-month period[2],[3]. The risk of resistance is increased for ongoing or prophylactic therapy, which should be administered only under supervision by a physician.
Drug-resistant thrush and treatment failure
Although reports of azole -resistant candidiasis are of concern[5], the nature of fungal organisms is such that occurrence of drug resistance is lower than that of bacterial and viral infections. C. albicans resistance is very low and treatment failure is typically a result of poor adherence to treatment; incorrect use of treatment or misdiagnosis/differential diagnosis; or that another strain of Candida (e.g. C. glabrata) may be the cause[1],[5]. Referral for additional investigation (e.g. vaginal specimen for microscopy, culture and sensitivity, pH testing) is advised. Boric acid 600mg capsules (unlicensed), inserted intravaginally (not taken orally), once or twice daily for 14 days have shown some success in the treatment of resistant vaginal candidiasis[10],[19].
For complicated cases, multiple doses of treatment may be required[1]. For all women, pharmacists should advise seeking further medical attention if the symptoms do not resolve within 7–14 days.
Thrush in men
Although thrush is often thought of as a female condition, it can also affect men and may be present without causing any symptoms[20]. Asymptomatic men, including those who may have engaged in sexual activity with a woman who has thrush, do not require treatment[3],[9],[21],[22]. Symptomatic men who present with thrush usually experience redness, discharge and irritation at the head of the penis and under the foreskin[20],[23]. As with women, the discharge looks like cottage cheese; however, an unpleasant smell may also be present[20],[23]. Treatment is the same as that for women; topical creams and oral capsules containing an azole are usually recommended for uncomplicated, acute thrush[20].
Lifestyle and self-management
Various lifestyle factors may contribute to the development of thrush and the discomfort associated with its symptoms. Men and women with thrush should be advised to avoid using irritants (e.g. soaps and fragranced shower gels) in the genital area when showering[20]. Pharmacists and healthcare professionals should recommend fragrance-free and soap-free alternatives, and that showers are preferable to baths[20],[23]. Both men and women should also be advised to keep their genitals clean and dry as Candida species, like many fungi, grow in damp, moist environments[20],[23]. Loose-fitting cotton underwear is also recommended[23].
Although most people do not acquire uncomplicated thrush through sex[3], patients should avoid sex or use condoms (if engaging in sexual intercourse) when thrush symptoms are present[23].
Dietary changes or adjuvant use of probiotics have been noted to increase the rate of clinical cure and absence of abnormal laboratory results, as well as short-term relapse within one month[24]. However, the effectiveness of probiotics is strain dependent.
Box 3 describes a common scenario of a female presenting in a community pharmacy requesting advice about thrush.
Box 3: Scenario of a patient presenting in a pharmacy asking about treatment for thrush
You are a recently registered pharmacist completing a locum shift in a small chain pharmacy in a rural location in the UK. A woman aged around 30 years enters the pharmacy and asks to speak privately with the pharmacist. She asks you to recommend the best treatment for thrush.
Upon questioning the patient, you find out that:
- She has had the symptoms for two days;
- She describes the discharge as white and not particularly smelly, but says it’s “a bit itchy down there”;
- She had this once before, a few years ago when she was pregnant. At that time, she used a cream and it went away in around a week;
- She takes the combined oral contraceptive pill, with no changes in brand or strength recently.
She wants to know the best way to treat her symptoms.
Based on the information provided, you conclude the likely diagnosis is uncomplicated thrush. Therefore, you decide that appropriate treatment could consist of either topical or oral azole therapy.
You explain this to her, highlighting that if she chooses a topical treatment she should apply the night-time dose prior to bed to increase the contact time. In addition, with both formulations, she must complete the course of treatment, despite symptoms resolving or the occurrence of menstruation.
On discussion, the patient prefers to take 150mg of fluconazole orally as a stat dose. You explain that if vulvar symptoms are of concern, she could also apply topical azoles as adjuvant therapy.
She asks if her partner needs to be treated and you explain that the evidence of transfer from female to male in uncomplicated, non-recurrent thrush indicates that this is not necessary.
Finally, you explain that regardless of choice of pharmaceutical therapy, she should:
- Avoid using soaps and fragranced shower gels in the genital area;
- Take showers instead of baths;
- Dry the affected area properly;
- Wear loose-fitting cotton underwear;
- Avoid sex, or use condoms, until symptoms have cleared.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
Supported by Bayer
Bayer provided financial support in the production of this content. The authors were not paid to write this article and The Pharmaceutical Journal retained full editorial control at all times.
References
[1] National Institute for Health and Care Excellence. Candida – female genital. Available at: https://cks.nice.org.uk/candida -female-genital#!topicsummary (accessed August 2018)
[2] Rosa MI, Silva BR, Pires PS et al. Weekly fluconazole therapy for recurrent vulvovaginal candidiasis: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2013;167(2):132–136. doi: 10.1016/j.ejogrb.2012.12.001
[3] Workowski KA & Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64(3). PMID: 26042815
[4] Rathod SD & Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Women’s Health 2014;14(1):43. doi: 10.1186/1472-6874-14-43
[5] Sobel JD. Genital candidiasis. In: Sexually Transmitted Infections and Sexually Transmitted Diseases. 2011, Springer. p. 613–624.
[6] Ravel J, Gajer P, Abdo Z et al . Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 2011;108(1):4680–4687. doi: 10.1073/pnas.1002611107
[7] Gajer P, Brotman RM, Bai G et al . Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012;4(132):132ra52. doi: 10.1126/scitranslmed.3003605
[8] Anderson MR, Klink K & Cohrssen A. Evaluation of vaginal complaints. JAMA 2004;291(11):1368–1379. doi: 10.1001/jama.291.11.1368
[9] Rang HP, Dale MM, Ritter JM & Flower RJ. Antifungal drugs. In: Rang & Dale’s Pharmacology. 2016, Elsevier. p. 653–657.
[10] Clinical Effectiveness Group, British Association of Sexual Health and HIV. United Kingdom national guideline on the management of vulvovaginal candidiasis. 2007. Available at: https://www.bashhguidelines.org/media/1155/united-kingdom-national-guideline-on-the-management-of-vulvovaginal -candidiasis.pdf (accessed August 2018)
[11] British National Formulary (online) 2018. BMJ Group and Pharmaceutical Press London. Available at: https://bnf.nice.org.uk/ (accessed August 2018)
[12] Compendium Electronic Medicines, Summary of product characteristics, in Timodine Cream. 2017. Available at: https://www.medicines.org.uk/emc/product/203/smpc (accessed August 2018)
[13] Watson MC, Grimshaw JM, Bond CM et al . Oral versus intra-vaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): a systematic review. BJOG 2002;109(1):85–95. doi: 10.1111/j.1471-0528.2002.01142.x
[14] Nurbhai M, Grimshaw J, Watson M et al . Oral versus intra-vaginal imidazole and triazole anti-fungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst Rev 2007(4):CD002845. doi: 10.1002/14651858.cd002845.pub2
[15] Kovac M, Mitic G & Kovac Z. Miconazole and nystatin used as topical antifungal drugs interact equally strongly with warfarin. J Clin Pharm Ther 2012;37(1):45–48. doi: 10.1111/j.1365-2710.2011.01246.x
[16] Thirion DJ & Zanetti LAF. Potentiation of warfarin’s hypoprothrombinemic effect with miconazole vaginal suppositories. Pharmacotherapy 2000;20(1):98–99. PMID: 10641982
[17] Aguin TJ & Sobel JD. Vulvovaginal candidiasis in pregnancy. Curr Infect Dis Rep 2015;17(6):30. doi: 10.1007/s11908 -015-0462-0
[18] Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 2016;214(1):15–21. doi: 10.1016/j.ajog.2015.06.067
[19] Chew SY & Than LTL. Vulvovaginal candidosis: contemporary challenges and the future of prophylactic and therapeutic approaches. Mycoses 2016;59(5):262–273. doi: 10.1111/myc.12455
[20] NHS Inform. Thrush in men. 2018. Available at: https://www.nhsinform.scot/illnesses-and-conditions/infections-and-poisoning/thrush-in-men (accessed August 2018)
[21] Fong IW. The value of treating the sexual partners of women with recurrent vaginal candidiasis with ketoconazole. Genitourin Med 1992;68(3):174–176. PMID: 1607194
[22] Bisschop MP, Merkus JM, Scheygrond H & van Cutsem J. Co-treatment of the male partner in vaginal candidosis: a double-blind randomized control study. Br J Obstet Gynaecol 1986;93(1):79–81. PMID: 3510660
[23] NHS Choices. Thrush in men and women. 2017. Available at: https://www.nhs.uk/conditions/thrush-in-men-and-women/ (accessed August 2018)
[24] Xie HY, Feng D, Wei DM et al. Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst Rev 2017;(11):CD010496. doi: 10.1002/14651858.CD010496.pub2


