Courtesy of Jonathan Buisson
Around 1,500 pharmaceutical companies are at risk of failure to comply with the Falsified Medicines Directive (FMD) because they have yet to start working with the European Medicines Verification Organisation (EMVO), the body has warned.
The EMVO said that just 841 companies have completed the first stage of connection to the EU Hub — the EU-wide database at the centre of the FMD — which is the beginning of a process known as ‘on-boarding’. It added that it can take up to six months to complete the process, ahead of the February 2019 deadline for completion.
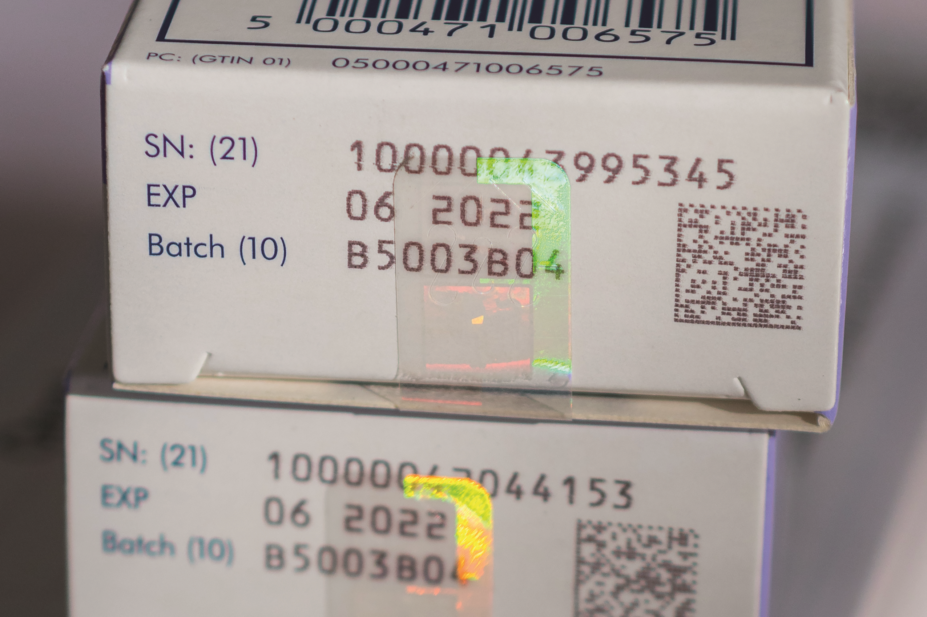
Under the FMD, more than 2,000 pharmaceutical firms with marketing authorisations to supply prescription medicines to the European Economic Area must connect to the Hub and upload to it unique identification data for each pack of medicine they manufacture or repackage.
In a letter to pharmaceutical companies dated 6 August 2018, the EMVO said that of 2,291 companies that need to connect to the Hub, only 841 companies have completed the first stage of connection: the signing of a participation agreement. Of those, only 106 companies have progressed to become fully connected to the production environment of the EU Hub.
“In January 2018, EMVO announced that the very last opportunity to on-board timely is June 2018,” the letter said.
“Any on-boarding after that date may entail a risk to the company’s compliance with the FMD and consequently to the business too.”
The letter warns companies that the on-boarding process can take up to six months to complete, and that as of the date of the letter, there are only 131 working days before FMD comes into force. It urges companies who have yet to start on-boarding to begin the process as soon as possible, and states that EMVO will not be held responsible or liable for any consequences to companies that may arise from late on-boarding, or failure to on-board.


