Science Photo Library
The Competition and Markets Authority (CMA) is consulting on proposals from Essential Pharma, the manufacturer of Priadel, to maintain supply of the bipolar disorder medicine to the UK for five years.
This follows pricing negotiations between the manufacturer and the government, which have seen the cost of Priadel tablets more than double.
The proposals are part of a set of commitments put forward to the CMA by the manufacturer, following the launch of an investigation into “suspected anti-competitive practices” in October 2020.
If the proposals are accepted following the consultation, the CMA’s investigation will close.
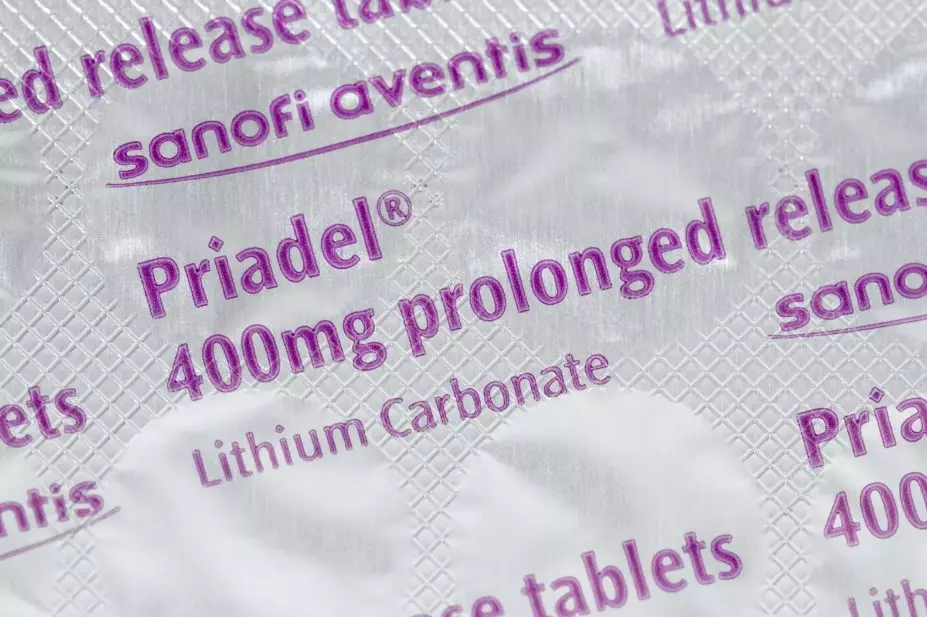
The CMA opened its investigation after Essential Pharma announced plans to stop producing Priadel 400mg and 200mg tablets, which the watchdog said may have “abused a dominant position in relation to lithium-based medicines for treating bipolar disorder”.
“If Priadel was withdrawn, this would require patients to switch to alternative, more expensive treatments, such as Camcolit, which is also owned by Essential Pharma,” said the CMA.
Priadel 400mg and 200mg were previously priced at £4.02 and £2.76 per pack of 100, respectively, with Camcolit 400mg costing £48.18 per pack of 100 and Essential Pharma’s generic lithium carbonate 250mg priced at £87 per pack of 100.
However, after the investigation began, Essential Pharma paused the withdrawal of Priadel and entered into price negotiations with the Department of Health and Social Care (DHSC).
They agreed to increase the price of Priadel 400mg and 200mg tablets to £8.50 and £7.50 per pack of 100, respectively — more than double the previous prices.
According to the NHS Business Services Authority’s dictionary of medicines and devices, these prices came into effect on 9 November 2020.
Essential Pharma also proposed formal commitments to the CMA to address competition concerns, which would last for five years from the date of their acceptance.
These include continuing “to ensure appropriate and continued supplies of Priadel to the UK on terms agreed from time to time with the DHSC,” and committing to “not serv[ing] a discontinuation notice to the DHSC relating to Priadel”.
In addition, any potential divestment of the supply of Priadel “shall be made on terms that ensure that Priadel continues to be supplied in the UK until the end of the period of five years”.
Essential Pharma will also be expected to notify the CMA if it plans to divest itself of Priadel.
“The CMA’s preliminary view is that the proposed commitments meet its competition concerns and [it] is now seeking views from others before accepting them formally,” said the CMA in a statement on 24 November 2020. “If accepted, the commitments will bring the investigation to an end.”
A spokesperson for Essential Pharma said it welcomes “the decision by the CMA to close its investigation, subject to acceptance of our commitments”.
“We have been supplying Priadel at a loss to the UK for a number of years, and following our constructive dialogue with the DHSC, the resulting improved pricing now allows us to supply Priadel in the UK on a sustainable, commercially viable basis,” they told The Pharmaceutical Journal.
“Essential Pharma is committed to its founding principle of providing access to low volume drugs, which otherwise may not be available on a sustainable basis, at a fair price. This swift outcome is good for the NHS and will give certainty to patients using this important product for years to come.”
Ann Pope, the CMA’s senior director of antitrust, added: “Since the CMA intervened just last month, Essential Pharma has agreed to carry on supplying Priadel at a price agreed with the DHSC, which we hope will give peace of mind to the thousands of patients who rely on it.
“We will carefully consider any responses to the consultation on the proposed commitments offered by Essential Pharma before reaching our final decision, with the best protection for patients in mind.”
Concerns around the additional cost of switching patients from Priadel to an alternative lithium carbonate medicine were previously raised by the Royal Pharmaceutical Society (RPS), which wrote to the DHSC, alongside nine other health bodies, calling for the government to intervene in the withdrawal.
Sandra Gidley, president of the RPS, said: “We’re delighted that negotiations on the price of Priadel have come to this positive conclusion for patients with bipolar disorder. They will now be able to remain on the drug that keeps them stable.”
The consultation will run until 9 December 2020.


