Dr P Marazzi / Science Photo Library
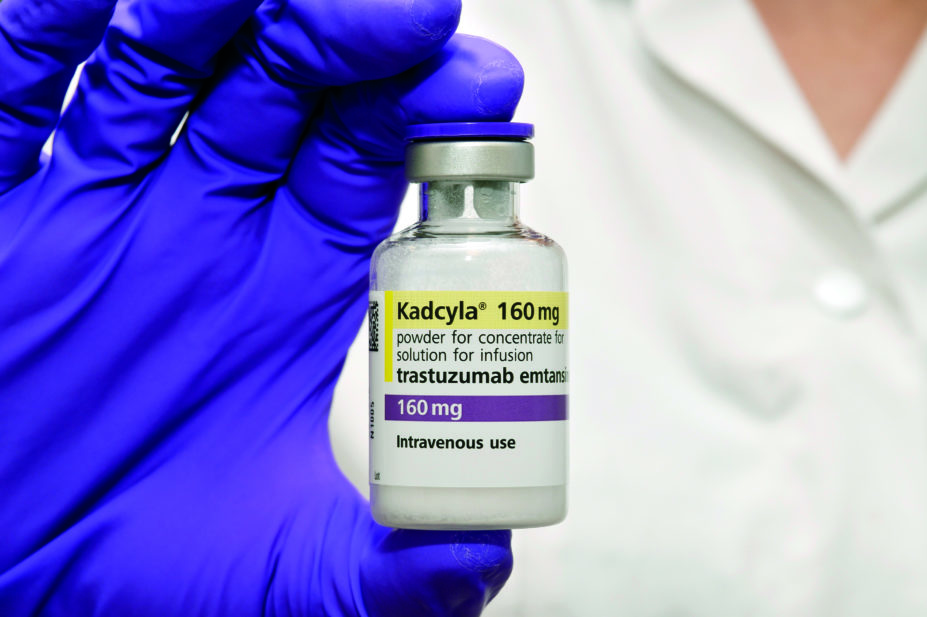
NHS Scotland has approved the use of Kadcyla, a drug used to treat HER2-positive breast cancer, which makes it one of 18 countries to offer the drug. In December 2016, the National Institute for Health and Care Excellence (NICE) recommended Kadcyla to be withdrawn for use on the NHS in England on the grounds that, at £90,000 per year per patient, its benefits did not justify its cost.
As part of a campaign called ‘Unlock Kadcyla’, led by the charity Breast Cancer Now and four women with incurable secondary breast cancer, more than 13,000 people signed a petition which was presented to the Scottish Medicines Consortium (SMC) and Roche (the company that manufactures Kadcyla). Consequently, the decision was made to make the drug available in Scotland.
The treatment options for this type of breast cancer at this time are relatively limited. The SMC says that the drug will enable patients to spend more time with their families and, in some cases, return to work.
“This decision will transform treatment options for women with HER2 positive secondary breast cancer in Scotland,” says Mary Allison, director of the charity, which aims to eliminate death from the disease during the next few decades.
“Both the Scottish government and Breast Cancer Now share the same vision of making sure that by 2050 everyone who develops breast cancer will live.
“If we are to achieve this, we’ll need to ensure that patients in Scotland are able to access the best possible treatments – and today is a real step forward for women with HER2 positive disease.”