DR P. MARAZZI / SCIENCE PHOTO LIBRARY
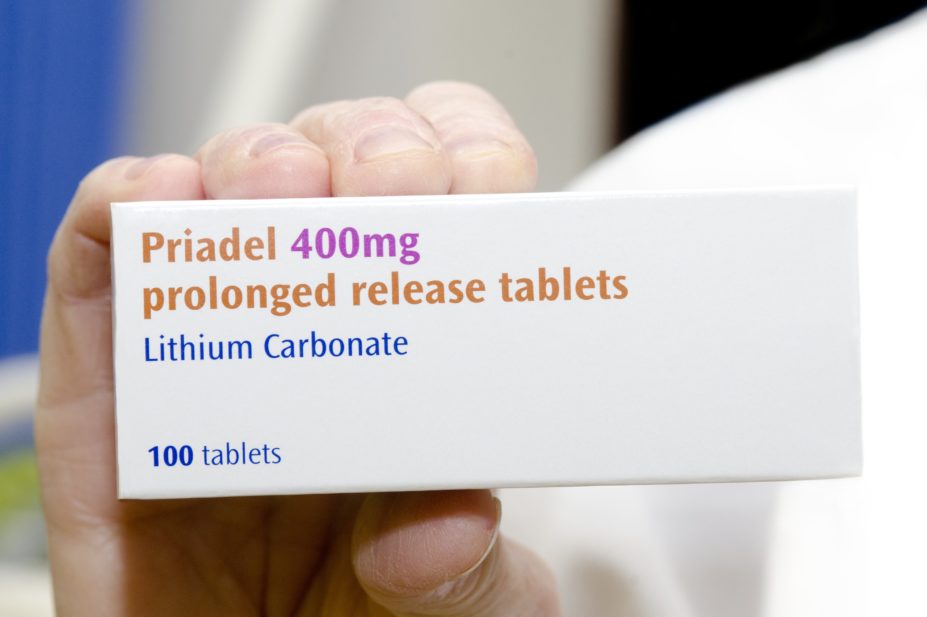
The Competition and Markets Authority (CMA) has launched an investigation into a manufacturer’s decision to discontinue the commonly prescribed first-line treatment for bipolar disorder, Priadel.
The manufacturer, Essential Pharma, is being investigated for “suspected anti-competitive practices” after it announced plans to stop producing Priadel 200mg and 400mg, with supplies expected to run out by April 2021.
Following the CMA’s announcement, the company has agreed to pause the withdrawal of Priadel while the CMA investigates.
The CMA said in a statement on 6 October 2020 that it will investigate whether the company “abused a dominant position in relation to lithium-based medicines for treating bipolar disorder, which it sells under the brand names ‘Priadel’ and ‘Camcolit’, by proposing to withdraw the supply of Priadel to UK patients”.
“The withdrawal of Priadel would mean that thousands of patients need to switch to alternative, more expensive, lithium treatments, such as Camcolit.”
Priadel 400mg and 200mg are currently priced at £4.02 and £2.76 per pack of 100, respectively. Meanwhile, Camcolit 400mg (Essential Pharma) costs £48.18 per pack of 100 and Essential Pharma’s generic lithium carbonate 250mg costs £87 per pack of 100.
The other lithium carbonate alternative that is available, Liskonum, which is owned by Teopharma, costs £11.84 per pack of 100.
Concerns around the additional cost of switching patients from Priadel to an alternative lithium carbonate medicine were previously raised by the Royal Pharmaceutical Society (RPS), which wrote to the Department of Health and Social Care (DHSC), alongside nine other health bodies, calling for the government to intervene in the withdrawal.
Commenting on the investigation, Sandra Gidley, president of the RPS, said: “The thousands of people who rely on Priadel will welcome the news that the government and CMA have listened to our concerns about the need to maintain patient access to this vital medicine.”
She added that the DHSC and Essential Pharma “must now work together to ensure this temporary pause in the withdrawal leads to a long-term solution”.
Ingvild Liborg, chief executive officer of Essential Pharma, said providing sustainable supply of low-volume, established products “is not possible when they are loss-making and pricing restrictions do not always provide for viable solutions”.
“We hope that the decision we have made to withdraw our discontinuation notice for Priadel will enable us to re-engage in a productive and constructive dialogue with the Department of Health and Social Care (DHSC) on Priadel, which we sell at a loss in the UK and at a price lower than in other European markets,” he said.
“We look forward to working with both the CMA and the DHSC towards a positive outcome that, in particular, results in the sustainable supply of Priadel to patients in the UK.”
Andrea Coscelli, chief executive of the CMA, said she welcomed the company’s decision to continue supply during the investigation.
“Thousands of people across the UK rely on lithium-based drugs to manage bipolar disorder, so it’s important that we protect their interests by scrutinising potential competition concerns to reach a fair conclusion as quickly as possible,” she said.
The CMA statement noted that no decision has been made as to whether the law has been broken, but said the CMA has reasonable grounds to suspect that Essential Pharma may have infringed the Chapter II prohibition of the Competition Act 1998.
Companies found to have infringed this prohibition could be forced to pay a financial penalty of up to 10% of their annual worldwide group turnover.
You may also be interested in

Warwick Smith: ‘The pandemic has removed factors that allowed us to be resilient for Brexit’

Health bodies make ‘urgent’ call to Matt Hancock to intervene in the proposed withdrawal of bipolar drug