Shutterstock
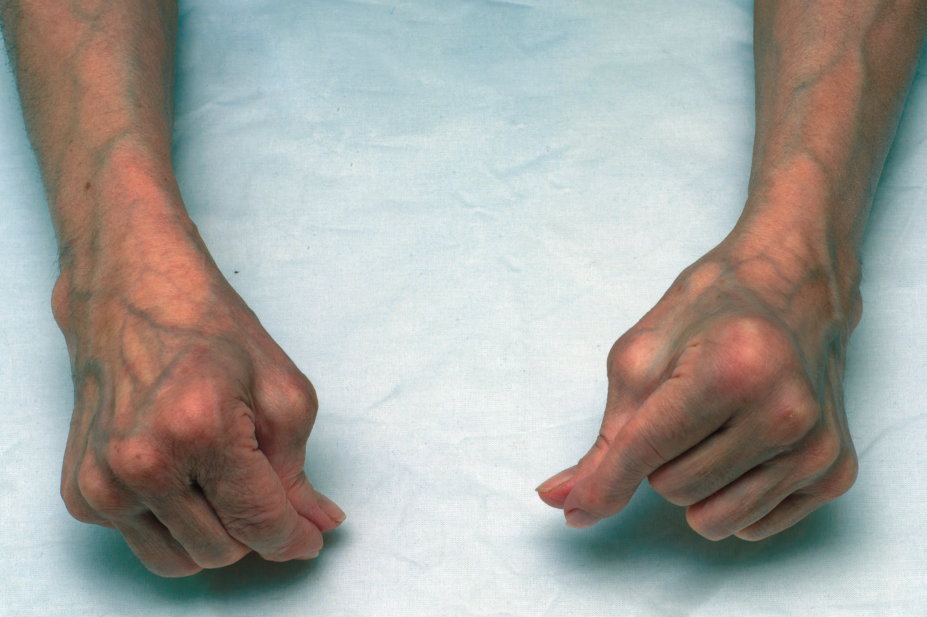
The European Medicines Agency (EMA)’s Committee for Medicinal Products for Human Use (CHMP) has recommended that baricitinib (Olumiant; Eli Lilly), an oral therapy for rheumatoid arthritis (RA), is granted a marketing authorisation in the EU.
Unlike biologic therapies for RA, which consist of large proteins that must be delivered by injection or infusion, baricitinib is a small molecule drug that can be administered orally.
The drug, which is taken alone or in combination with methotrexate, reversibly inhibits two of the four known JAK enzymes. These enzymes are activated when cytokines bind to cell-surface receptors and many mediators involved in autoimmune inflammation, including some interleukins and interferons, signal via the JAK family in this way.
Responses to RA therapies vary greatly between patients and only around half achieve a satisfactory response under existing therapies. As such, Olivia Belle, director of external affairs at charity Arthritis Research UK, says there is a need to get new treatments to patients as quickly as possible.
“Delays in accessing effective treatments can cause frustration and increase the risk of joint damage and long-term disability,” she says.
“It’s important that we continue to discover and develop new treatment therapies so we can get the right treatment to the right patient at the right time, helping more people with rheumatoid arthritis push back the limits of arthritis.”
The CHMP’s opinion was based on data from four randomised controlled trials involving a total of 3,100 adults with moderate-to-severe active RA. Two trials compared baricitinib with placebo, one compared it with methotrexate and another with adalimumab (Humira; Abbvie), an injectable monoclonal antibody.
All four trials showed that baricitinib was more effective at reducing disease activity compared with methotrexate and adalimumab. In placebo-controlled trials, the drug showed efficacy in patients who had not responded to biologic disease-modifying anti-rheumatic drugs as well as in treatment-naive patients.
The most common side effects reported in the trials were increased blood lipid levels, upper respiratory tract infection and nausea.
The CHMP recommendation indicates that baricitinib is used in adults with moderate-to-severe RA who have had an inadequate response or are intolerant to at least one disease-modifying anti-rheumatic drug. It will be available at doses of 2mg and 4mg.
The approval would make it the first JAK inhibitor to be licensed for RA in the EU. The condition results in chronic inflammation leading to irreversible joint damage, joint pain and stiffness, and fatigue.
Another JAK inhibitor for RA, tofacitinib (Xeljanz; Pfizer), is marketed in 45 countries including the United States, but was denied marketing authorisation in 2013 by the EMA, which cited “major concerns” over the treatment’s safety profile.
The EMA’s concerns relate to the risks of serious infection resulting from immunosuppression, as well as concerns about the risk of certain cancers and gastrointestinal perforations, which the CHMP said it was not clear could be managed in clinical practice.
Pfizer refiled is application for tofacitinib to the EMA earlier in 2016 with additional clinical trial and open-label data.


