Shutterstock.com
The European Medicines Agency (EMA) is recommending a raft of new measures to help educate patients and carers on the proper use of adrenaline auto-injectors.
The move follows a review by the EMA’s Committee for Medicinal Products for Human Use (CHMP) of evidence relating to the devices, which are used to inject adrenaline in patients suffering potentially fatal severe allergic reactions (anaphylaxis).
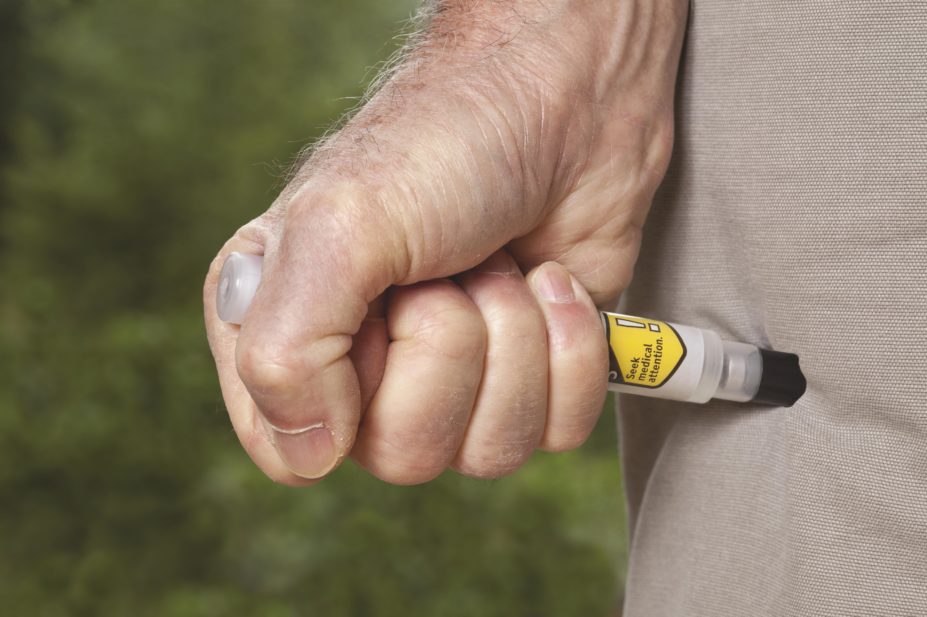
The review was prompted by concerns that adrenaline auto-injectors used in the UK may deliver adrenaline into the skin rather than into muscle, as they are intended to, potentially altering absorption of the drug.
The new CHMP review concludes that intramuscular injection is the preferred method for administering adrenaline in order to obtain a rapid response. It also notes that several factors influence whether adrenaline is actually delivered into a muscle; these include needle length, the thickness of fat under the skin, the precise auto-injector mechanisms, the angle at which the device is placed on the skin and the force applied by the user.
The CHMP says that training patients and carers is essential to optimising use of the devices. Accordingly, it will ask the companies that market the devices to develop more effective educational materials for patients and healthcare professionals.
Recommended measures include dummy devices for practising injections; audiovisual materials to demonstrate how to use the devices; a checklist for prescribers to ensure patients are given sufficient information on how to use their auto-injector; and updated product information with a recommendation that patients should carry two auto-injectors at all times.