Jynto / Wikimedia Commons
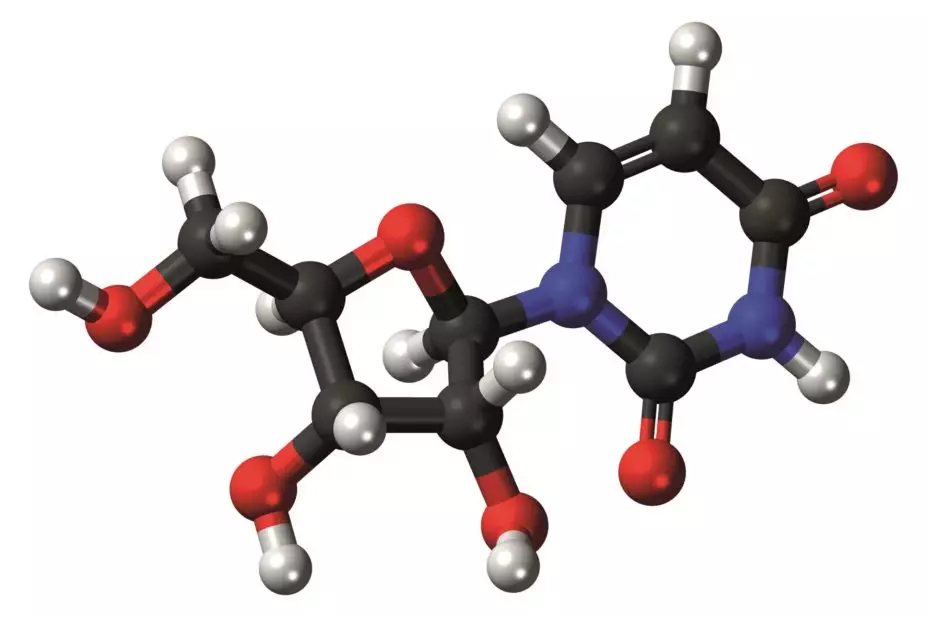
The US Food and Drug Administration (FDA) has approved uridine triacetate (Xuriden) for the treatment of orotic aciduria — a rare genetic hereditary metabolic disorder that affects just 20 patients worldwide.
It is the first FDA -approved drug for this condition, caused by a defective or deficient enzyme that prevents the body from synthesizing uridine, which is a necessary component of ribonucleic acid.
Patients with the condition have abnormal blood counts and urinary tract obstruction. They can also fail to thrive and face developmental delays.
Uridine triacetate has been given FDA orphan drug status because of the rarity of the condition. The drug review was also fast-tracked by the FDA because it has the potential to offer major advances in treatment.
The drug manufacturer, Wellstat Therapeutics Corporation, was also granted a FDA rare paediatric disease priority review voucher, which encourages the development of new drugs and biologics for the prevention and treatment of rare paediatric diseases.
The decision follows the results of a six-week open label trial in four patients with the condition aged 3–19 years old, and a six-month trial extension.
The results both at six weeks and six months revealed that the hematologic parameters were stable. No side effects were found for up to nine months.
The European Medicines Agency (EMA) — the drugs’ safety watchdog for the European Union (EU) — said uridine triacetate does not currently have a central authorisation in the EU. “This medicine is also not currently under evaluation for marketing authorisation by the EMA and there is currently no orphan designation for uridine triacetate in hereditary orotic aciduria,” it said.