Shutterstock.com
A screening device that uses artificial intelligence (AI) to eliminate the need for a clinician to interpret results has been authorised for use for the first time in the United States.
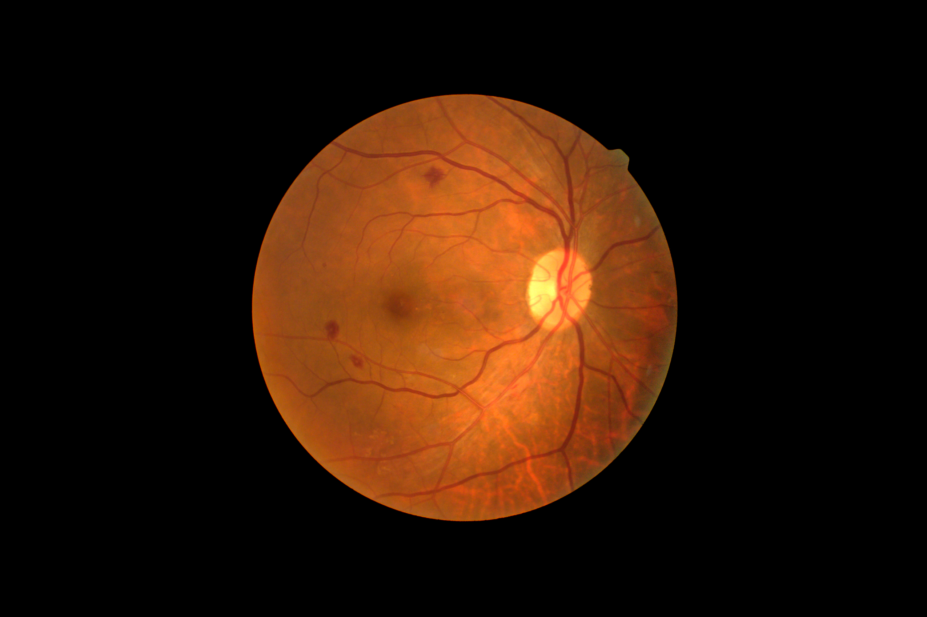
The US Food and Drug Administration has approved the marketing of a device that uses AI to determine whether a person with diabetes has more than mild diabetic retinopathy. It is the first time such a device has been approved and opens up the possibility of screening being done by healthcare providers who are not usually involved in eye care.
Digital images of the patient’s eyes are taken using a retinal camera and are then uploaded to a cloud server where the IDx-DR AI is installed. If the images are of sufficient quality, the software will produce one of two responses — that the patient has more than mild diabetic retinopathy, in which case they need to be referred to an eye care professional, or that they are negative for this but should be rescreened in 12 months.
Around half of people with diabetes in the United States do not see a specialist eye doctor each year, but the IDx-DR approach could allow them to be screened when seeing their primary care doctor, or potentially by other healthcare professionals.
In a 900-person trial, the software was found to correctly identify the presence of more than mild retinopathy 87.4% of the time, and patients who did not have more than mild retinopathy 89.5% of the time. The method is not suitable for some patients, including pregnant women or those with a history of eye surgery or injections.
More than 30 million people in the United States have diabetes and diabetic retinopathy is the most common cause of visions loss in this group.